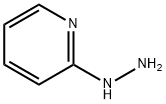
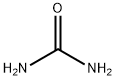
1,2,4-Triazolo[4,3-a]pyridin-3(2H)-one synthesis
- Product Name:1,2,4-Triazolo[4,3-a]pyridin-3(2H)-one
- CAS Number:6969-71-7
- Molecular formula:C6H5N3O
- Molecular Weight:135.12
109-09-1
563-41-7
![1,2,4-Triazolo[4,3-a]pyridin-3(2H)-one](/CAS/GIF/6969-71-7.gif)
6969-71-7
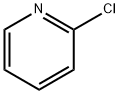
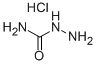
General procedure for the synthesis of [1,2,4]triazolo[4,3-a]pyridin-3-(2H)-one from 2-chloropyridine and aminourea hydrochloride: 2-chloropyridine (5.0 g, 44.03 mmol), aminourea hydrochloride (9.8 g, 88.06 mmol), and 2-ethoxyethanol (15 mL) were mixed and heated to reflux. Concentrated hydrochloric acid (0.1 mL dissolved in 1 mL of 2-ethoxyethanol) was slowly added to the reaction mixture. The reaction was kept at reflux for 24 hours and subsequently cooled to about 60 °C and diluted with deionized water (15 mL). The resulting light yellow solid product was collected by filtration and washed with deionized water to afford the title compound (2.2 g, 51% yield). The melting point of the product was 235-240°C. 1H NMR (300 MHz, DMSO-d6) δ 6.51-6.60 (m, 1H), 7.05-7.22 (m, 2H), 7.80 (d, J=7.1 Hz, 1H), 12.45 (br, 1H, exchangeable with D2O); IR (KBr) ν 3173, 3098. 3048, 1717, 1634, 1536, 1348 cm-1; MS 136 (M+1).
Yield:6969-71-7 75%
Reaction Conditions:
in neat (no solvent) for 0.0138889 h;Microwave irradiation;
Steps:
3.3.1. Preparation of 1,2,4-Triazolo[4,3-a]pyridin-3(2H)-one (1) from 7 under Microwave Conditions (Samsung M182DN; 300 W)
Firstly, 500 mg (4.58 mmol) g of 2-hydrazinopyridine (7) and 275 mg (4.58 mmol) or 550 mg(9.16 mmol) of urea were placed in a conical flask. Reactions were carried out under microwave radiation. The progress of the reaction was monitored by TLC (chloroform-methanol 9:1). After 50 s,15 cm3 of water was added to the mixture, after which the resulting product was filtered o on aBüchner funnel. The product was obtained with eciency 49% in an equimolar reaction and 75%by reaction with a two fold molar excess of urea; Rf = 0.62, HPLC 97.9% (reaction with molar excess),tM 1.95 min.
References:
Jaskowska, Jolanta;Zar eba, Przemys?aw;Sliwa, Pawe?;Pindelska, Edyta;Sata?a, Grzegorz;Majka, Zbigniew [Molecules,2019,vol. 24,# 8,art. no. 1609]
109-04-6
529 suppliers
$6.00/25g
![1,2,4-Triazolo[4,3-a]pyridin-3(2H)-one](/CAS/GIF/6969-71-7.gif)
6969-71-7
303 suppliers
$6.00/1g

109-09-1
0 suppliers
$10.60/1gm:
![1,2,4-Triazolo[4,3-a]pyridin-3(2H)-one](/CAS/GIF/6969-71-7.gif)
6969-71-7
303 suppliers
$6.00/1g