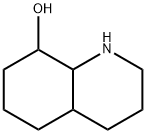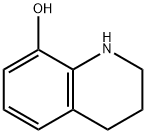
1,2,3,4-Tetrahydro-8-hydroxyquinoline synthesis
- Product Name:1,2,3,4-Tetrahydro-8-hydroxyquinoline
- CAS Number:6640-50-2
- Molecular formula:C9H11NO
- Molecular Weight:149.19
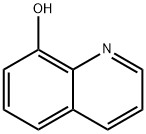
148-24-3

6640-50-2
The general procedure for the synthesis of 8-hydroxy-1,2,3,4-tetrahydroquinoline from 8-hydroxyquinoline was as follows: 100 mmol of 8-hydroxyquinoline and polymer microspheres Poly (DVB-co-NVP) loaded with 0.7 mmol of rhodium catalyst were added to 500 ml of water and the mixture was transferred to a reactor. The air in the reaction kettle was replaced five times with hydrogen and then filled with hydrogen to atmospheric pressure. The reaction was stirred at room temperature (25°C) and atmospheric pressure (1 atm) for 40 minutes. Upon completion of the reaction, aqueous phase extraction was performed three times, each time using ether as the extraction solvent. All ether extracts were combined and the ether was removed by rotary evaporator to give dry 8-hydroxy-1,2,3,4-tetrahydroquinoline in 95% yield.
130-16-5
429 suppliers
$5.00/5g

6640-50-2
101 suppliers
$9.00/250mg
Yield: 72%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in tetrahydrofuran;methanol at 20; under 760.051 Torr; for 24.5 h;Solvent;
Steps:
1,2,3,4-Tetrahydro-8-quinolinol (Compound 3)
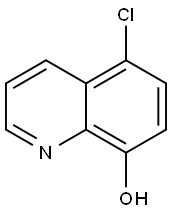
Compound 1a (5-chloro-8-quinolinol: 1.26 g, 7.00 mmol)was dissolved in 14 mL of a mixture containing THF andMeOH (1 : 1), added to 10% Pd/C (744.9 mg, 0.70 mmol), andhydrogenated at 1 atm in H2 gas at room temperature. Afterbeing stirred for 24.5 h, the reaction mixture was filtered,and the filtrate was concentrated under reduced pressure. Thecrude product was purified by recrystallization with CHCl3 toafford compound 3 (724.0 mg, 72%) as a pale orange crystal.Analytical data on compound 3 was as follows: 1H-NMR(CD3OD, 400 MHz) δ: 2.05-2.15 (m, 2H), 2.89 (t, J = 6.1 Hz,2H), 3.41-3.49 (m, 2H), 6.78 (d, J = 7.5 Hz, 1H), 6.82 (d,J = 8.1 Hz, 1H), 7.20 (dd, J = 8.1, 7.5 Hz, 1H); 13C-NMR(CDCl3 + MeOH-d4 5 drop, 100 MHz) δ: 19.5, 24.9, 42.5,113.8, 118.1, 120.5, 129.3, 132.2, 150.7; HR-MS (EI) Calcd forC9H11NO ([M]+): 149.0841. Found: 149.0836.
References:
Karuo, Yukiko;Kawai, Kentaro;Nakahara-Yamada, Mayuko;Nishimura, Hitoshi;Omote, Masaaki;Sato, Kazuyuki;Shiraki, Riona;Tarui, Atsushi;Tsunokawa, Ryo;Yoshida, Ayaka [Chemical and Pharmaceutical Bulletin,2021,vol. 69,# 6,p. 557 - 563]

148-24-3
874 suppliers
$5.00/25g

6640-50-2
101 suppliers
$9.00/250mg
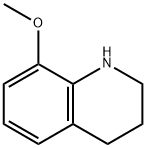
53899-17-5
41 suppliers
$430.00/1g

6640-50-2
101 suppliers
$9.00/250mg
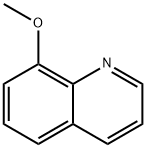
938-33-0
101 suppliers
$22.80/250mg

6640-50-2
101 suppliers
$9.00/250mg