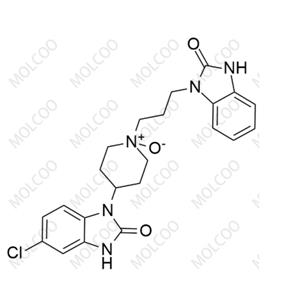
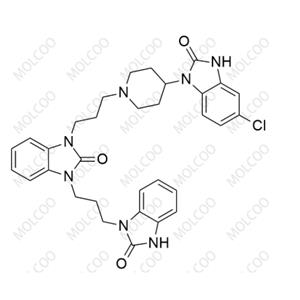
rac-Domperidone EP Impurity C NEW
| Price | Get Latest Price | ||
| Package | 10mg | 50mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 1000 |
| Update Time: | 2025-07-02 |
Product Details
| Product Name: rac-Domperidone EP Impurity C | CAS No.: 118435-03-3 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/02 |
| Molecular formula: C22H24ClN5O3 |
rac-Domperidone EP Impurity C;118435-03-3
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
1. Drug standards and drug impurity reference substances: provide more than 20,000 kinds of spot impurity reference substances, with sufficient supply and same-day delivery. We have a professional R&D team and comprehensive quality control testing to ensure product quality and reliability.
2. Customized synthesis of impurities and new molecules: Quickly respond to the customized needs of CDE impurities, stably supply impurities that have established quality standards, and provide customized synthesis services for new compounds in the research and development of innovative drugs.
3. Preparation and separation of unknown impurities: With a professional impurity preparation and separation technical team and SFC preparation and separation instruments, we can solve the problems of impurity preparation in complex projects.
4. Process development of new drug intermediates: provide the supply of new drugs and new molecular impurities, process optimization services, as well as the screening and impurity analysis of commercialization routes.
5. Peptide synthesis: Provide customized peptide synthesis services, and cover all kinds of degradation impurities and process impurities generated in the research and development of peptide drugs. At the same time, it provides comprehensive structural confirmation data and customized testing services.
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $29.00/200mg |
VIP1Y
|
TargetMol Chemicals Inc.
|
2025-07-26 | |
| $29.00/200mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2025-07-16 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-02-10 |
INQUIRY







 China
China