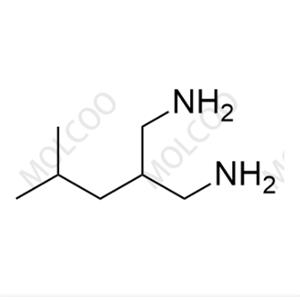
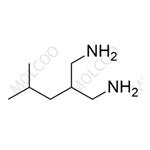
Pregabalin Impurity NEW
| Price | Get Latest Price | ||
| Package | 10g | 20g | 50g |
| Min. Order: | 10g |
| Supply Ability: | 5000 |
| Update Time: | 2025-07-31 |
| Related documents: | COA |
Product Details
| Product Name: Pregabalin Impurity | CAS No.: 159029-27-3 |
| Min. Order: 10g | Purity: 99% |
| Supply Ability: 5000 | Release date: 2025/07/31 |

Product Overview
Pregabalin impurity reference standards, as crucial tools in drug research and quality control, feature high purity and accuracy, making them a preferred choice in the field of pharmaceutical science. Our range of pregabalin impurity reference standards, including PD0224377, L, and others, cater to various client needs.
Product Advantages
High Purity: Our pregabalin impurity reference standards boast a purity level above 95%, ensuring accurate and reliable experimental results.
Multiple Types: We offer a variety of impurity types to meet clients' needs at different stages of research and development.
Excellent Service: We provide professional technical support and after-sales service, ensuring that clients receive timely assistance and answers during use.
Application Scenarios
Pregabalin impurity reference standards are widely used in drug research and development, quality control, pharmacodynamics evaluation, and other fields. In drug research, they can be used for impurity analysis, purity determination, and structural identification. In quality control, they help ensure the stability and safety of drug products.
Contact Information
For any questions or requirements, please feel free to contact us. We will be delighted to provide you with high-quality products and services.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $100.00/50kg |
VIP1Y
|
HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
|
2024-08-09 | |
| $1.00/1kg |
VIP1Y
|
Shaanxi Xianhe Biotech Co., Ltd
|
2024-12-17 | |
| $0.00/1KG |
VIP1Y
|
Forest chemical technology
|
2025-01-14 |







 China
China