
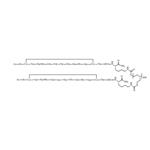
Pegcetacoplan(APL-2) NEW
| Price | $820 |
| Package | 1g |
| Min. Order: | 10g |
| Supply Ability: | 100000000kg |
| Update Time: | 2025-07-25 |
Product Details
| Product Name: Pegcetacoplan(APL-2) | CAS No.: 2019171-69-6 |
| Min. Order: 10g | Purity: 99% |
| Supply Ability: 100000000kg | Release date: 2025/07/25 |
Introduction
Pegcetacoplan is the first medication approved for treating paroxysmal nocturnal hemoglobinuria (PNH). It binds to and inhibits the complement protein C3. Pegcetacoplan received approval for medical use in the United States in May 2021. The U.S. Food and Drug Administration (FDA) designates it as a first-in-class drug.
Mechanism of Action
Pegcetacoplan binds to complement protein C3 and its activation fragment C3b, thereby regulating the cleavage of C3 and the generation of downstream effectors of complement activation. Acting proximally in the complement cascade, it controls both C3b-mediated extravascular hemolysis and terminal complement-mediated intravascular hemolysis.
Indications
Treatment of paroxysmal nocturnal hemoglobinuria (PNH).
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/100mg |
VIP2Y
|
Xian confluore Biological Technology Co., Ltd.
|
2024-04-01 | |
| $0.00/1G |
VIP1Y
|
Shenzhen Nexcon Pharmatechs Ltd.
|
2025-05-30 | |
| $50.00/1kg |
VIP6Y
|
Hebei Yanxi Chemical Co., Ltd.
|
2023-02-07 | |
| $2.00/100kg |
VIP1Y
|
ZHENGZHOU JIUYI TIME NEW MATERIALS CO,.LTD
|
2025-06-23 |
- Since: 2021-07-13
- Address: Room 925, Building 1, No. 225 Xinggong Road, Tinglin Town, Jinshan District, Shanghai
+86-13917132737
lily.xiang@wayxbio.com






 China
China