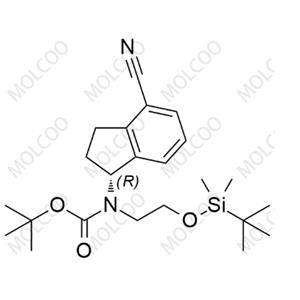
Ozanimod Impurity 41 NEW
| Price | Get Latest Price | ||
| Package | 10mg | 50mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 1000 |
| Update Time: | 2025-07-18 |
Product Details
| Product Name: Ozanimod Impurity 41 | CAS No.: 1306763-41-6 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/18 |
| Molecular formula: C23H36N2O3Si |
Ozanimod Impurity 41

WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com.
Product Information
Product Number: O036041
English Name: Ozanimod Impurity 41
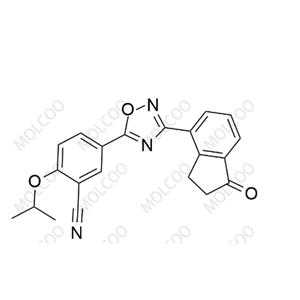
English Alias: (R)-tert-butyl (2-((tert-butyldimethylsilyl)oxy)ethyl)(4-cyano-2,3-dihydro-1H-inden-1-yl)carbamate
CAS Number: 1306763-41-6
Molecular Formula: C??H??N?O?Si
Molecular Weight: 416.63
Advantages
Well-defined and distinct structure: Contains tert-butyl carbamate, TBS ether, and 4-cyanoindane moieties, differing from ozanimod intermediates by retained protecting groups. It can be accurately identified via HPLC and GC-MS as a specific marker for impurity detection;
High stability and traceability: Synergistic effects of silyl ether and carbamate ensure stability under non-acidic conditions. As a by-product of incomplete deprotection in ozanimod synthesis, it directly reflects deprotection efficiency, improving process tracing accuracy;
High detection sensitivity: Unique retention behavior from cyano polarity and silyl hydrophobicity, combined with indane UV absorption (240-260nm), enables trace analysis via HPLC-UV or GC-MS, compatible with silyl-protected amine detection systems.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to quantify Ozanimod Impurity 41 in API production, ensuring residual protected impurities meet quality standards post-deprotection;
Synthesis optimization: Optimizing TBS/carbamate deprotection conditions (e.g., acid concentration, time) by monitoring impurity levels to enhance intermediate purity and reduce downstream interference;
Intermediate quality assessment: Evaluating chiral intermediate purity in ozanimod synthesis to support efficiency of subsequent cyclization and amination reactions.
Background Description
Research Status
Analytical method development: Validating UPLC-DAD assays with optimized mobile phases for baseline separation of impurity and target intermediate, achieving 0.5 ppm detection limits;
Deprotection kinetics: Studying impurity degradation under varying acid conditions to clarify factors governing synchronous TBS/Boc removal;
Chiral purity validation: Developing chiral HPLC methods to confirm stereochemical consistency with ozanimod, ensuring no enantiomeric interference;
Scale-up process control: Implementing impurity monitoring in kg-scale production to optimize deprotection parameters for consistent quality.
Product Information
Product Number: O036041
English Name: Ozanimod Impurity 41
English Alias: (R)-tert-butyl (2-((tert-butyldimethylsilyl)oxy)ethyl)(4-cyano-2,3-dihydro-1H-inden-1-yl)carbamate
CAS Number: 1306763-41-6
Molecular Formula: C??H??N?O?Si
Molecular Weight: 416.63
Advantages
Well-defined and distinct structure: Contains tert-butyl carbamate, TBS ether, and 4-cyanoindane moieties, differing from ozanimod intermediates by retained protecting groups. It can be accurately identified via HPLC and GC-MS as a specific marker for impurity detection;
High stability and traceability: Synergistic effects of silyl ether and carbamate ensure stability under non-acidic conditions. As a by-product of incomplete deprotection in ozanimod synthesis, it directly reflects deprotection efficiency, improving process tracing accuracy;
High detection sensitivity: Unique retention behavior from cyano polarity and silyl hydrophobicity, combined with indane UV absorption (240-260nm), enables trace analysis via HPLC-UV or GC-MS, compatible with silyl-protected amine detection systems.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to quantify Ozanimod Impurity 41 in API production, ensuring residual protected impurities meet quality standards post-deprotection;
Synthesis optimization: Optimizing TBS/carbamate deprotection conditions (e.g., acid concentration, time) by monitoring impurity levels to enhance intermediate purity and reduce downstream interference;
Intermediate quality assessment: Evaluating chiral intermediate purity in ozanimod synthesis to support efficiency of subsequent cyclization and amination reactions.
Background Description
Research Status
Analytical method development: Validating UPLC-DAD assays with optimized mobile phases for baseline separation of impurity and target intermediate, achieving 0.5 ppm detection limits;
Deprotection kinetics: Studying impurity degradation under varying acid conditions to clarify factors governing synchronous TBS/Boc removal;
Chiral purity validation: Developing chiral HPLC methods to confirm stereochemical consistency with ozanimod, ensuring no enantiomeric interference;
Scale-up process control: Implementing impurity monitoring in kg-scale production to optimize deprotection parameters for consistent quality.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $41.00/1mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2025-07-15 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-16 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-04-02 |







 China
China