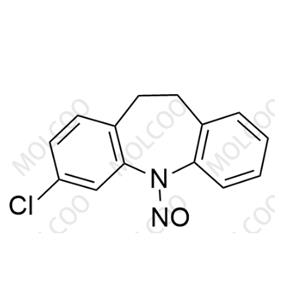
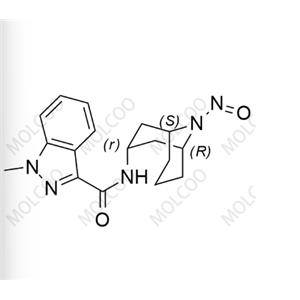
N-Nitroso Clomipramine EP Impurity F NEW
| Price | Get Latest Price | ||
| Package | 10mg | 50mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 1000 |
| Update Time: | 2025-08-01 |
Product Details
| Product Name: N-Nitroso Clomipramine EP Impurity F | CAS No.: 78213-40-8 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/08/01 |
| Molecular formula: C14H11ClN2O |
N-Nitroso Clomipramine EP Impurity F
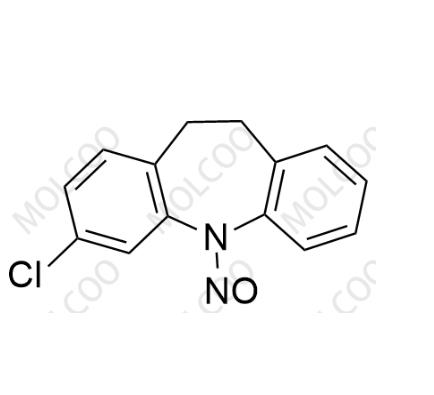
roduct Information
Product Number: C102009
English Name: N-Nitroso Clomipramine EP Impurity F
English Alias: 3-chloro-5-nitroso-10,11-dihydro-5H-dibenzo[b,f]azepine
CAS Number: 78213-40-8
Molecular Formula: C??H??ClN?O
Molecular Weight: 258.70
Advantages
Well-defined with distinct functional groups: Contains dibenzo[b,f]azepine core, 3-chloro substituent, 5-nitroso (-N-NO), and 10,11-dihydro structure. Unlike clomipramine (tricyclic antidepressant with 5-aminomethyl side chain), its nitroso polarity, chlorine electronegativity, and tricyclic hydrophobicity create significant differences, enabling precise differentiation via HPLC/TLC as a specific marker;
High stability and traceability: Rigid dibenzoazepine structure and stability of chlorine/nitroso ensure stability under dark, low-temperature conditions. As a derivative from 5-amino nitrosation during storage/degradation, it directly reflects amino stability and nitrite exposure, improving impurity tracing accuracy;
High detection sensitivity: Tricyclic conjugation shows strong UV absorption (250-290nm), combined with m/z 259 [M+H]? enabling ppb-level analysis via LC-MS, compatible with tricyclic antidepressant nitroso impurity systems.
Applications
Pharmaceutical quality control: Used as an EP reference standard to quantify N-Nitroso Clomipramine EP Impurity F in APIs, ensuring compliance with EP limits for this nitroso impurity;
Stability studies: Monitoring impurity levels under varying conditions (pH, light) to assess degradation trends and support shelf-life assurance;
Impurity profile analysis: Identifying 5-amino nitrosation as a key impurity source to guide process optimization (e.g., nitrite control).
Background Description
Research Status
Analytical method validation: Developing UPLC assays with C18 columns for separation, achieving 0.05 ppb detection limits;
Nitrosation mechanism: Studying impurity formation kinetics under varying nitrite concentration and pH to clarify 5-amino-to-nitroso conversion pathways;
Control strategies: Using nitrosation inhibitors (e.g., sulfites) to keep impurity levels below EP limits (<0.01%);
Toxicity evaluation: Conducting in vitro genotoxicity tests (e.g., chromosome aberration) to assess hazards and support EP limit revisions.
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP1Y
|
Guangzhou Weiaokang Pharmaceutical Technology Co., Ltd
|
2025-08-01 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-16 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-03-18 |








 China
China