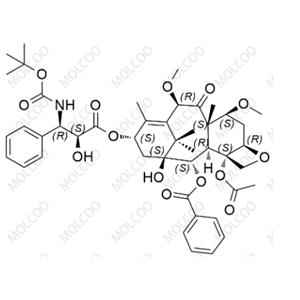
Cabazitaxel Impurity NEW
| Price | Get Latest Price | ||
| Package | 10mg | 50mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 1000 |
| Update Time: | 2025-07-17 |
Product Details
| Product Name: Cabazitaxel Impurity | CAS No.: 1714967-27-7 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/17 |
| Molecular formula: C45H57NO14 |
Cabazitaxel Impurity;1714967-27-7

WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com.
Product Information
Product Number: C100008
English Name: Cabazitaxel Impurity 8
English Alias: (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-acetoxy-9-(((2S,3R)-3-((tert-butoxycarbonyl)amino)-2-hydroxy-3-phenylpropanoyl)oxy)-11-hydroxy-4,6-dimethoxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate
CAS Number: 1714967-27-7
Molecular Formula: C??H??NO??
Molecular Weight: 835.93
Advantages
Well-defined and Stable Structure: Its complex and precise chemical structure, along with good stability, helps in deeply analyzing the by-product formation mechanisms of multiple reactions during cabazitaxel synthesis, such as acylation, hydroxyl protection and deprotection, thus optimizing the production process.
Reliable Detection Standard: Containing multiple functional groups like ester, hydroxyl, amino groups and a special fused - ring structure, it can provide an accurate standard for detection methods such as HPLC and LC-MS, greatly improving the separation and quantification accuracy of complex impurities of cabazitaxel.
Facilitating Quality Research: It can assist in studying the influence of impurity structure on drug stability, efficacy and toxicological properties, providing key evidence for formulating scientific and reasonable impurity control strategies to ensure drug quality and safety.
Applications
Drug Development: During the research and development of cabazitaxel and its formulations, it is used as an impurity reference standard to identify and quantify Cabazitaxel Impurity 8, accurately evaluating the purity of APIs and formulations to ensure drug quality meets the standards.
Quality Control: Serving as a standard substance, it is used to verify the sensitivity and specificity of detection methods such as HPLC and LC-MS, strictly controlling the content of this impurity during production to meet pharmacopoeia and relevant regulatory requirements.
Stability Studies: Investigating the degradation behavior of this impurity under different environmental conditions such as light, high temperature and high humidity, evaluating its impact on the stability of cabazitaxel formulations, and providing strong data support for determining reasonable storage conditions and shelf life.
Background Description
Research Status
Detection Method Optimization: Using advanced technologies such as ultra-high-performance liquid chromatography - tandem mass spectrometry (UPLC-MS/MS) and high-resolution mass spectrometry (HRMS), continuously developing and improving highly sensitive and selective detection methods to achieve trace detection of this impurity.
Synthesis Process Improvement: Deeply studying the formation pathway of this impurity, and developing efficient synthesis processes to reduce impurity generation by optimizing reaction conditions (such as reaction temperature, time, catalyst selection), improving raw material ratios and reaction sequences.
Toxicological Evaluation: Using in vitro cytotoxicity experiments, animal models and other means to comprehensively evaluate the potential toxicity of this impurity, providing sufficient data support for scientifically formulating reasonable impurity limit standards.
Stability Research: Systematically studying the stability of this impurity under the influence of different environmental factors, analyzing its interactions with other components in cabazitaxel formulations, and further improving the drug quality control system
Product Information
Product Number: C100008
English Name: Cabazitaxel Impurity 8
English Alias: (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-acetoxy-9-(((2S,3R)-3-((tert-butoxycarbonyl)amino)-2-hydroxy-3-phenylpropanoyl)oxy)-11-hydroxy-4,6-dimethoxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate
CAS Number: 1714967-27-7
Molecular Formula: C??H??NO??
Molecular Weight: 835.93
Advantages
Well-defined and Stable Structure: Its complex and precise chemical structure, along with good stability, helps in deeply analyzing the by-product formation mechanisms of multiple reactions during cabazitaxel synthesis, such as acylation, hydroxyl protection and deprotection, thus optimizing the production process.
Reliable Detection Standard: Containing multiple functional groups like ester, hydroxyl, amino groups and a special fused - ring structure, it can provide an accurate standard for detection methods such as HPLC and LC-MS, greatly improving the separation and quantification accuracy of complex impurities of cabazitaxel.
Facilitating Quality Research: It can assist in studying the influence of impurity structure on drug stability, efficacy and toxicological properties, providing key evidence for formulating scientific and reasonable impurity control strategies to ensure drug quality and safety.
Applications
Drug Development: During the research and development of cabazitaxel and its formulations, it is used as an impurity reference standard to identify and quantify Cabazitaxel Impurity 8, accurately evaluating the purity of APIs and formulations to ensure drug quality meets the standards.
Quality Control: Serving as a standard substance, it is used to verify the sensitivity and specificity of detection methods such as HPLC and LC-MS, strictly controlling the content of this impurity during production to meet pharmacopoeia and relevant regulatory requirements.
Stability Studies: Investigating the degradation behavior of this impurity under different environmental conditions such as light, high temperature and high humidity, evaluating its impact on the stability of cabazitaxel formulations, and providing strong data support for determining reasonable storage conditions and shelf life.
Background Description
Research Status
Detection Method Optimization: Using advanced technologies such as ultra-high-performance liquid chromatography - tandem mass spectrometry (UPLC-MS/MS) and high-resolution mass spectrometry (HRMS), continuously developing and improving highly sensitive and selective detection methods to achieve trace detection of this impurity.
Synthesis Process Improvement: Deeply studying the formation pathway of this impurity, and developing efficient synthesis processes to reduce impurity generation by optimizing reaction conditions (such as reaction temperature, time, catalyst selection), improving raw material ratios and reaction sequences.
Toxicological Evaluation: Using in vitro cytotoxicity experiments, animal models and other means to comprehensively evaluate the potential toxicity of this impurity, providing sufficient data support for scientifically formulating reasonable impurity limit standards.
Stability Research: Systematically studying the stability of this impurity under the influence of different environmental factors, analyzing its interactions with other components in cabazitaxel formulations, and further improving the drug quality control system
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $89.00/100mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2025-06-12 | |
| $1.00/1g |
VIP5Y
|
RongNa Biotechnology Co.,Ltd
|
2025-06-02 | |
| $0.00/1kg |
VIP2Y
|
Shaanxi TNJONE Pharmaceutical Co., Ltd
|
2024-04-30 |









 China
China