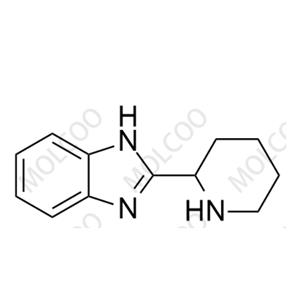
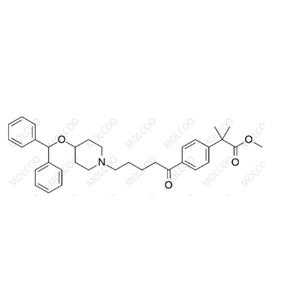
Bilastine Impurity NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 100000 |
| Update Time: | 2025-06-18 |
Product Details
| Product Name: Bilastine Impurity | CAS No.: 51785-23-0 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 100000 | Release date: 2025/06/18 |
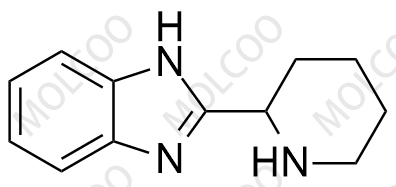
Bilastine impurity reference standards are crucial substances used in drug development, quality control, and project approval processes. These impurity reference standards cover various impurities that may exist in bilastine drugs, playing a vital role in ensuring the purity and stability of the drugs.
Product Information
Product Name: Bilastine Impurity Reference Standards
CAS Numbers: Including but not limited to 202189-78-4, 2069238-47-5 (specific CAS numbers vary depending on the type of impurity)
Molecular Formula: C28H37N3O3 (the molecular formula of specific impurities may differ)
Molecular Weight: 463.6117 (the molecular weight of specific impurities may differ)
Purity: Generally above 98%, with some reaching over 99%
Storage Conditions: Recommended to be stored in a low-temperature environment at 2-8°C to maintain stability
Usage: Primarily used for impurity analysis, quality control, and new drug approval processes during drug development
Product Features
Comprehensive Range: Offers a complete set of bilastine impurity reference standards to meet various needs in drug development
High Purity: The purity of impurity reference standards is generally above 98%, ensuring the accuracy of analysis results
Stable and Reliable: Undergoes strict quality control to ensure the stability and reliability of the impurity reference standards
Application Areas
Bilastine impurity reference standards are widely used in drug development, quality control departments of pharmaceutical companies, drug testing institutions, and new drug approval processes. They provide important reference standards for impurity analysis, purity detection, and stability studies during drug development.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-24 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-06-28 | |
| $39.00/25mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2025-07-15 |





 China
China