- Ro 5126766
-

- $52.00 / 1mg
-
2025-07-15
- CAS:946128-88-7
- Min. Order:
- Purity: 98.81%
- Supply Ability: 10g
- RO5126766(CH5126766)
-

- $1.00 / 1KG
-
2019-07-06
- CAS:946128-88-7
- Min. Order: 1KG
- Purity: 98-100%
- Supply Ability: 1000kg
|
| | RO5126766(CH5126766) Basic information |
| Product Name: | RO5126766(CH5126766) | | Synonyms: | RO5126766(CH5126766);RO5126766(CH5126766) 3-[[2-[(Methylaminosulfonyl)amino]-3-fluoropyridin-4-yl]methyl]-4-methyl-7-[(pyrimidin-2-yl)oxy]-2H-1-benzopyran-2-one;3-[[2-[(Methylaminosulfonyl)amino]-3-fluoropyridin-4-yl]methyl]-4-methyl-7-[(pyrimidin-2-yl)oxy]-2H-1-benzopyran-2-one;CS-1469;3-{2-(methylaminosulfonyl)amino-3-fluoropyridin-4-ylmethyl}-4-methyl-7-(pyrimidin-2-yloxy)-2-oxo-2H-1-benzopyran;Sulfamide, N-[3-fluoro-4-[[4-methyl-2-oxo-7-(2-pyrimidinyloxy)-2H-1-benzopyran-3-yl]methyl]-2-pyridinyl]-N'-methyl-;CH5126766;3-[(2-((N-methylsulfamoyl)amino)-3-fluoropyridin-4-yl)methyl]-4-methyl-7-(pyrimidin-2-yloxy)-chromen-2-one,3-{2-(methylaminosulfonyl)amino-3- | | CAS: | 946128-88-7 | | MF: | C21H18FN5O5S | | MW: | 471.46 | | EINECS: | | | Product Categories: | | | Mol File: | 946128-88-7.mol | 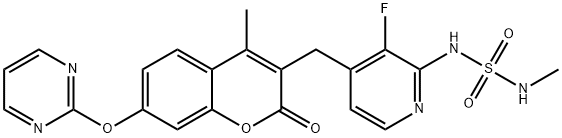 |
| | RO5126766(CH5126766) Chemical Properties |
| Boiling point | 690.8±65.0 °C(Predicted) | | density | 1.495±0.06 g/cm3(Predicted) | | storage temp. | Store at -20°C | | solubility | insoluble in H2O; insoluble in EtOH; ≥35.7 mg/mL in DMSO | | form | Powder | | pka | 6.60±0.50(Predicted) | | color | White to yellow | | InChI | InChI=1S/C21H18FN5O5S/c1-12-15-5-4-14(31-21-25-7-3-8-26-21)11-17(15)32-20(28)16(12)10-13-6-9-24-19(18(13)22)27-33(29,30)23-2/h3-9,11,23H,10H2,1-2H3,(H,24,27) | | InChIKey | LMMJFBMMJUMSJS-UHFFFAOYSA-N | | SMILES | S(NC1=NC=CC(CC2=C(C)C3=CC=C(OC4=NC=CC=N4)C=C3OC2=O)=C1F)(NC)(=O)=O |
| | RO5126766(CH5126766) Usage And Synthesis |
| Uses | Avutometinib (Ro 5126766) is a first-in-class dual MEK/RAF inhibitor that allosterically inhibits BRAFV600E, CRAF, MEK, and BRAF (IC50: 8.2, 56, 160 nM, and 190 nM, respectively). | | Definition | ChEBI: CH5126766 is a member of the class of coumarins that is 4-methyl-7-[(pyrimidin-2-yl)oxy]coumarin carrying an additional [2-[(methylaminosulfonyl)amino]-3-fluoropyridin-4-yl]methyl substituent at position 3. It has a role as an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor and an antineoplastic agent. It is an aryloxypyrimidine, a member of coumarins, a member of pyridines, an organofluorine compound and a member of sulfamides. | | Biological Activity | ro5126766 (ch5126766) is a first-in-class dual inhibitor of raf/mek [1].the ras/raf/mek/erk signaling pathway is an important signal transduction system and participates in cell differentiation, movement, division and death. activated ras activates raf kinase, which then phosphorylates and activates mek (mek1 and mek2) [1]. the mutations in braf, ras, and nf1 are associated with many human tumors [2].ro5126766 (ch5126766) is a first-in-class dual raf/mek inhibitor. in cell-free kinase assays, ch5126766 effectively inhibited the phosphorylation of mek1 protein by raf and the activation of erk2 protein by mek1 with ic50 values of 0.0082-0.056 and 0.16 μm, respectively. in nci-h460 (kras q61h) human lung large cell carcinoma cell line, ro5126766 induced cell-cycle inhibitor p27kip1 protein expression and caused g1 arrest. in hct116 kras-mutant colorectal cancer cells, ro5126766 ch5126766 completely inhibited the phosphorylation of mek and erk [2].in japanese patients with advanced solid tumors, ro5126766 exhibited the maximum tolerable dose (mtd) of 2.25 mg/day once daily [1]. in a hct116 (g13d kras) mouse xenograft model, ro5126766 (1.5 mg/kg) inhibited perk and erk signaling and exhibited ed50 value of 0.056 mg/kg [2]. | | in vivo | In KRAS-mutant xenograft models, Avutometinib (Ro 5126766) inhibits growth and causes tumor regressions more effectively than another allosteric MEK inhibitor, PD0325901. Preclinical data from a series of human tumor mouse xenograft models indicates an ED50 for Ro 5126766 of 0.03 to 0.23 mg/kg and an ED90 of 0.15 to 1.56 mg/kg. These effective doses are associated with target trough concentrations of 17 to 133 ng/L and 87 to 901 ng/mL, respectively. [1]. In this experiment, Avutometinib or PD0325901 is administrated at their maximum tolerated dose (MTD) in the HCT116 model (1.5 and 25 mg/kg, respectively). These doses inhibit pERK and ERK signaling output at similar degrees in the tumors from the drug-treated mice at 4 hours from the first drug administration. Moreover, in HCT116 models, the ED50 for Avutometinib and PD0325901 are 0.056 and 0.80 mg/kg, respectively. Therefore, the doses used for this experiment are 26.8- and 31.3-fold higher doses than the 50% effective doses, respectively. Daily oral administration of either drug causes significant tumor regression of each these tumors. However, whereas inhibition of tumor growth is maintained for the entire 28-day treatment period in Avutometinib-treated mice, tumor models receiving PD0325901 become refractory after 10 days of treatment[3]. | | IC 50 | MEK: 160 nM (IC50); BRafV600E: 8.2 nM (IC50); Braf: 190 nM (IC50); CRAF: 56 nM (IC50) | | references | [1]. honda k, yamamoto n, nokihara h, et al. phase i and pharmacokinetic/pharmacodynamic study of ro5126766, a first-in-class dual raf/mek inhibitor, in japanese patients with advanced solid tumors. cancer chemother pharmacol, 2013, 72(3): 577-584.
[2]. ishii n, harada n, joseph ew, et al. enhanced inhibition of erk signaling by a novel allosteric mek inhibitor, ch5126766, that suppresses feedback reactivation of raf activity. cancer res, 2013, 73(13): 4050-4060. |
| | RO5126766(CH5126766) Preparation Products And Raw materials |
|