6-Methyl-3piperidinecarboxylic acid Methyl ester manufacturers
|
| | 6-Methyl-3piperidinecarboxylic acid Methyl ester Basic information |
| Product Name: | 6-Methyl-3piperidinecarboxylic acid Methyl ester | | Synonyms: | 6-Methyl-3piperidinecarboxylic acid Methyl ester;ethyl 6-Methylpiperidine-3-carboxylate;Methyl 6-Methylpiperidine-3-carboxylate;3-Piperidinecarboxylic acid, 6-methyl-, methyl ester;6-Methyl-piperidinecarboxylic acid Methyl ester;Methyl 6-methylpiperidine-3-carboxylate - [M9067] | | CAS: | 908245-03-4 | | MF: | C8H15NO2 | | MW: | 157.21 | | EINECS: | | | Product Categories: | | | Mol File: | 908245-03-4.mol | 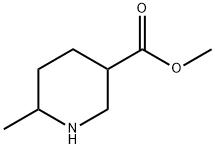 |
| | 6-Methyl-3piperidinecarboxylic acid Methyl ester Chemical Properties |
| Boiling point | 205.9±33.0 °C(Predicted) | | density | 0.982±0.06 g/cm3(Predicted) | | storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C | | pka | 9.47±0.10(Predicted) | | Appearance | Colorless to light yellow Liquid | | Optical Rotation | 0.40°(C=0.01g/ml CHCL3) |
| | 6-Methyl-3piperidinecarboxylic acid Methyl ester Usage And Synthesis |
| Synthesis | The general procedure for the synthesis of methyl 6-methyl-3-piperidinecarboxylate from methyl 6-methylnicotinate was as follows: methanol (300 mL), concentrated hydrochloric acid (13.0 g), 10% Pd/C catalyst (4.0 g), and methyl 6-methylnicotinate (20.0 g, 132 mmol) were added to a 400 mL Fisher-Porter reactor. The mixture was heated to 80 °C and stirred at 60 psi hydrogen pressure for 21 hours. Upon completion of the reaction, the mixture was cooled and filtered to remove the catalyst. The filtrate was evaporated under reduced pressure to afford methyl 6-methyl-3-piperidinecarboxylate (27.0 g, quantitative yield). The structure of the product was confirmed by 1H NMR to be consistent with the target compound. | | References | [1] Patent: US2010/93706, 2010, A1. Location in patent: Page/Page column 34
[2] Patent: EP3248980, 2017, A1. Location in patent: Paragraph 0163; 0164; 0165
[3] Patent: WO2010/124055, 2010, A1. Location in patent: Page/Page column 39-40
[4] Patent: WO2011/26917, 2011, A1. Location in patent: Page/Page column 93
[5] Patent: WO2011/26904, 2011, A1. Location in patent: Page/Page column 93 |
| | 6-Methyl-3piperidinecarboxylic acid Methyl ester Preparation Products And Raw materials |
|