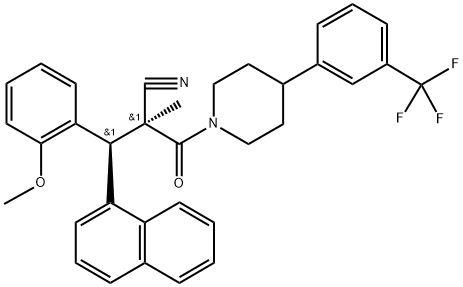
WAY-204688
- CAS No.
- 796854-35-8
- Chemical Name:
- WAY-204688
- Synonyms
- SIM-688;WAY-204688;(2S,3S)-3-(2-Methoxyphenyl)-2-methyl-3-(naphthalen-1-yl)-2-(4-(3-(trifluoromethyl)phenyl)piperidine-1-carbonyl)propanenitrile;1-Piperidinepropanenitrile, α-[(S)-(2-methoxyphenyl)-1-naphthalenylmethyl]-α-methyl-β-oxo-4-[3-(trifluoromethyl)phenyl]-, (αS)-
- CBNumber:
- CB53175601
- Molecular Formula:
- C34H31F3N2O2
- Molecular Weight:
- 556.63
- MDL Number:
- MFCD30532597
- MOL File:
- 796854-35-8.mol
| Boiling point | 708.7±60.0 °C(Predicted) |
|---|---|
| Density | 1.231±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | Solid |
| pka | -1.66±0.40(Predicted) |
| color | White to off-white |
| FDA UNII | QT9VF4MW6U |
WAY-204688 Chemical Properties,Uses,Production
Uses
WAY-204688 is an estrogen receptor (ER-α) selective, orally active inhibitor of NF-κB transcriptional activity with an IC50 of 122?±?30 nM for NF-κB-luciferase (NF-κB-luc) in HAECT-1 cells.
in vivo
WAY-204688 (5 mg/kg per day, po daily for 5 weeks) is evaluated in vivo for the ability to inhibit four proinflammatory genes (MHC, invariant chain (MHI), VCAM-1, RANTES, and TNF-α). The effect of WAY-204688 on induction of the gene products and on uterine wet weight is compared to that of 17α-ethinyl 17β-estradiol (EE at 10 μg/kg per day) in the same paradigm. Further characterization of WAY-204688 is carried out in several preclinical models of inflammatory disease. In the Lewis rat adjuvant-induced arthritis model (AIA), WAY-204688 is active at a dose of 0.3 mg/kg per day, po[1].
IC 50
NF-κB-luc: 122 nM (IC50, in HAECT-1 cell); NF-κB
References
[1] Caggiano TJ, et al. Estrogen receptor dependent inhibitors of NF-kappaB transcriptional activation-1 synthesis and biological evaluation of substituted 2-cyanopropanoic acid derivatives: pathway selective inhibitors of NF-kappaB, a potential treatment for rheumatoid arthritis. J Med Chem. 2007 Nov 1;50(22):5245-8. DOI:10.1021/jm701013k
WAY-204688 Preparation Products And Raw materials
Raw materials
Preparation Products
WAY-204688 Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| TargetMol Chemicals Inc. | +17819995354 | marketing@targetmol.com | United States | 32354 | 58 |
| Shanghai EFE Biological Technology Co., Ltd. | 021-65675885 18964387627 | info@efebio.com | China | 9803 | 58 |
| ShangHai Biochempartner Co.,Ltd | 177-54423994 17754423994 | 2853530910@QQ.com | China | 7991 | 62 |
| Shanghai SuperLan Chemcial Technique Centre | 021-2022843681 15618226720 | lucy@atkchemical.com | China | 10017 | 58 |
| Chuzhou KeMail Chemical Technology Co., Ltd | 0550-5196001 15000891977 | wj520wjxby@126.com | China | 1941 | 55 |
| Shenzhen Polymeri Biochemical Technology Co., Ltd. | +86-400-002-6226 +86-13028896684; | sales@rrkchem.com | China | 57422 | 58 |
| ChemeGen(Shanghai) Biotechnology Co.,Ltd. | 18818260767 | sales@chemegen.com | China | 11218 | 58 |
| cjbscvictory | 13348960310 | 3003867561@qq.com | China | 10523 | 58 |
| Bide Pharmatech Ltd. | 400-1647117 13681763483 | product02@bidepharm.com | China | 62321 | 58 |