| Identification | Back Directory | [Name]
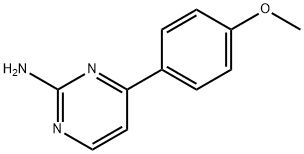
4-(4-METHOXYPHENYL)PYRIMIDIN-2-AMINE | [CAS]
99844-02-7 | [Synonyms]
BUTTPARK 16\04-19
4-(4-Methoxyphenyl)pyrimidin-2-
4-(4-Methoxyphenyl)pydimidin-2-amine
4-(4-METHOXYPHENYL)PYRIMIDIN-2-AMINE
4-(4-METHOXYPHENYL)-2-PYRIMIDINAMINE
2-ANIMO-4-(4-METHOXYPHENYL)PYRIMIDINE
2-AMINO-4-(4-METHOXYPHENYL)PYRIMIDINE
4-(4-METHOXYPHENYL)-2-PYRIMIDINYLAMINE
2-PyriMidinaMine, 4-(4-Methoxyphenyl)-
[4-(4-methoxyphenyl)pyrimidin-2-yl]amine
4-(4-Methoxyphenyl)-2-yl-pyriMidin-2-ylaMine
PyriMidine, 2-aMino-4-(p-Methoxyphenyl)- (6CI)
4-(4-METHOXYPHENYL)PYRIMIDIN-2-AMINE ISO 9001:2015 REACH
2-Amino-4-(4-methoxyphenyl)pyrimidine, 4-(2-Aminopyrimidin-4-yl)anisole | [Molecular Formula]
C11H11N3O | [MDL Number]
MFCD00665912 | [MOL File]
99844-02-7.mol | [Molecular Weight]
201.22 |
| Hazard Information | Back Directory | [Synthesis]
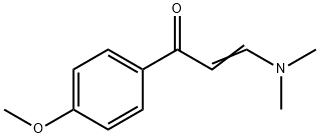
GENERAL METHOD: A mixture of 3-(dimethylamino)-1-(4-methoxyphenyl)prop-2-en-1-one (1 mmol), guanidine nitrate (2 mmol) and anhydrous K2CO3 (5 mmol) in n-butanol (10 mL) was refluxed for 12 hours. After completion of the reaction, it was cooled to room temperature and the reaction solution was poured into water (30 mL) and extracted with ethyl acetate (3 x 20 mL). The organic layers were combined and concentrated under reduced pressure, and the resulting crude product was purified by recrystallization from ether to give 4-(4-methoxyphenyl)pyrimidin-2-amine. | [References]
[1] Bioorganic and Medicinal Chemistry Letters, 2016, vol. 26, # 13, p. 3024 - 3028 |
|
|