| Identification | Back Directory | [Name]
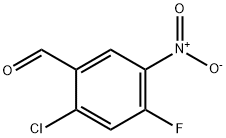
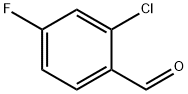
2-Chloro-4-fluoro-5-nitrobenzaldehyde | [CAS]
99329-85-8 | [Synonyms]
2-Chloro-4-fluoro-5-nitrobenzaldehy
2-Chloro-4-fluoro-5-nitrobenzaldehyde
Benzaldehyde, 2-chloro-4-fluoro-5-nitro- | [Molecular Formula]
C7H3ClFNO3 | [MDL Number]
MFCD17168722 | [MOL File]
99329-85-8.mol | [Molecular Weight]
203.55 |
| Chemical Properties | Back Directory | [Boiling point ]
303.6±42.0 °C(Predicted) | [density ]
1.576±0.06 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [Appearance]
White to off-white Solid | [InChI]
InChI=1S/C7H3ClFNO3/c8-5-2-6(9)7(10(12)13)1-4(5)3-11/h1-3H | [InChIKey]
HNOIAPRHNXBCAE-UHFFFAOYSA-N | [SMILES]
C(=O)C1=CC([N+]([O-])=O)=C(F)C=C1Cl |
| Hazard Information | Back Directory | [Uses]
2-Chloro-4-fluoro-5-nitrobenzaldehyde is a polysubstituted benzaldehyde analogue containing chlorine, fluorine and nitro active groups on its benzene ring structure, which can be replaced by other halogen atoms to synthesise new compounds. | [Synthesis]
General procedure for the synthesis of 2-chloro-4-fluoro-5-nitrobenzaldehyde from 2-chloro-4-fluorobenzaldehyde: 2-chloro-4-fluorobenzaldehyde (1.0 g, 6.3 mmol) was dissolved in concentrated sulphuric acid (8 ml) at 0 °C and protected by argon and stirred to form a homogeneous solution. Subsequently, potassium nitrate (0.70 g, 6.9 mmol) was added in batches and the reaction temperature was maintained at 0 °C for 30 min. After that, the temperature was slowly warmed up to room temperature and the warming process was controlled to be completed within 1.5 hours. After completion of the reaction, the reaction mixture was poured into a well-stirred ice/water mixture (100 ml) and extracted with ethyl acetate. The organic phases were combined, dried and concentrated to give 2-chloro-4-fluoro-5-nitrobenzaldehyde as a light yellow oil (1.15 g, 90% yield).1H NMR (CDCl3, 400 MHz) δ: 7.50 (1H, dd), 8.66 (1H, dd), 10.41 (1H, s). | [References]
[1] Patent: WO2007/36718, 2007, A2. Location in patent: Page/Page column 87 |
|
|