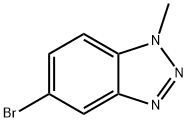
| Identification | Back Directory | [Name]
5-BroMo-1-Methyl-1H-benzo[d][1,2,3]triazole | [CAS]
944718-31-4 | [Synonyms]
5-Bromo-1-methylbenzotriazole
1H-Benzotriazole, 5-bromo-1-methyl-
5-bromo-1-methyl-1,2,3-benzotriazole
5-bromo-1-methyl-1H-1,2,3-benzotriazole
5-BroMo-1-Methyl-1H-benzo[d][1,2,3]triazole | [Molecular Formula]
C7H6BrN3 | [MDL Number]
MFCD19288764 | [MOL File]
944718-31-4.mol | [Molecular Weight]
212.047 |
| Chemical Properties | Back Directory | [Boiling point ]
332.6±15.0 °C(Predicted) | [density ]
1.76±0.1 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,2-8°C | [pka]
0.40±0.30(Predicted) | [Appearance]
Brown to reddish brown Solid |
| Hazard Information | Back Directory | [Synthesis]
General procedure for the synthesis of 5-bromo-1-methyl-1H-benzo[d][1,2,3]triazole from 4-bromo-N1-methylbenzene-1,2-diamine: 5-bromo-1-methyl-7H-benzo[2 (1.52 g, 22 mmol) was dissolved in water (5 ml) at 0 °C. The resulting solution was stirred at 0-10°C for 4 h. The pH was adjusted to 8 with potassium hydroxide (3N) and extracted with dichloromethane (2 x 200 ml). The organic layers were combined and concentrated in vacuum to give the residue. The residue was purified by silica gel chromatography (eluent: petroleum ether solution of 50% dichloromethane) to afford 5-bromo-1-methyl-1H-benzo[d][1,2,3]triazole as a red solid (1.5 g, 31% yield).LC/MS (ESI, m/z): [M + H]+ 213.1.1.1H-NMR (300 MHz, CDCl3) δ 8.24-8.25 (m, 1H), 7.60-7.63 (m, 1H), 7.42-7.46 (m, 1H), 4.32 (d, J = 5.4 Hz, 3H). | [References]
[1] Patent: WO2012/119046, 2012, A2. Location in patent: Page/Page column 207-208 |
|
|