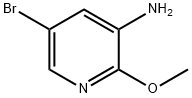
| Identification | Back Directory | [Name]
5-BROMO-2-METHOXY-3-CYANOPYRIDINE | [CAS]
884495-39-0 | [Synonyms]
5-BroMo-2-Methoxy-3-aMino...
5-Bromo-2-methoxypyridin-3-amine
5-bromo-6-methoxypyridin-4-amine
3-PyridinaMine,5-broMo-2-Methoxy-
5-BROMO-2-METHOXY-3-CYANOPYRIDINE
5-BROMO-2-METHOXY-3-AMINOPYRIDINE
2-methoxy-3-amino-5-bromopyridine
5-BroMo-2-(Methyloxy)-3-pyridinaMine
3-Amino-5-bromo-2-methoxypyridine 98%
3-Amino-5-bromo-2-methoxypyridine,96%
3-Amino-5-bromopyridin-2-yl methyl ether
5-BROMO-2-METHOXY-3-CYANOPYRIDINE ISO 9001:2015 REACH
5-Bromo-2-methoxypyridin-3-amine, 3-Amino-5-bromopyridin-2-yl methyl ether | [Molecular Formula]
C6H7BrN2O | [MDL Number]
MFCD07368887 | [MOL File]
884495-39-0.mol | [Molecular Weight]
203.04 |
| Chemical Properties | Back Directory | [Melting point ]
53-55℃ | [Boiling point ]
284.5±35.0 °C(Predicted) | [density ]
1.622±0.06 g/cm3(Predicted) | [storage temp. ]
Room temperature. | [pka]
1.98±0.20(Predicted) | [Appearance]
Off-white to light brown Solid | [Water Solubility ]
Slightly soluble in water. | [InChI]
InChI=1S/C6H7BrN2O/c1-10-6-5(8)2-4(7)3-9-6/h2-3H,8H2,1H3 | [InChIKey]
HJOOFLFWIISCAI-UHFFFAOYSA-N | [SMILES]
C1(OC)=NC=C(Br)C=C1N |
| Hazard Information | Back Directory | [Uses]
3-Amino-5-bromo-2-methoxypyridine is used as pharmaceutical intermediate. | [Synthesis]
General procedure for the synthesis of 3-amino-5-bromo-2-methoxypyridine from 5-bromo-2-methoxy-3-nitropyridine: To a solution of 5-bromo-2-methoxy-3-nitropyridine (19.0 g, 82 mmol) in ethyl acetate (300 mL) was added tin (II) chloride dihydrate (74 g, 328 mmol). The reaction mixture was stirred and refluxed for 3 hours (note: an exothermic phenomenon occurs at the beginning of the reaction, which gradually subsides after about 10 minutes, at which time the heating bath can be temporarily removed). After completion of the reaction, the reaction mixture was cooled to room temperature and concentrated under reduced pressure to a light yellow slurry. The slurry was poured into water (300 mL), 6 N sodium hydroxide solution (300 mL), ice (300 g), and dichloromethane (300 mL) were added, and stirred for 2 hours until most of the solids were dissolved. A small amount of insoluble material was removed by filtration, the organic phase was separated, dried over anhydrous sodium sulfate, filtered and evaporated to dryness. The residual brown oil was solidified by grinding with hexane, filtered and dried under vacuum to give the target product 3-amino-5-bromo-2-methoxypyridine (13.40 g, 81% yield) as a light green solid. Mass spectrum (electrospray ionization) m/e: 202.8 [M+H]+. | [References]
[1] Patent: US2014/134133, 2014, A1. Location in patent: Paragraph 0387
[2] Patent: WO2008/33854, 2008, A1. Location in patent: Page/Page column 74-75
[3] Patent: WO2014/22128, 2014, A1. Location in patent: Paragraph 0150
[4] Patent: US2014/234254, 2014, A1. Location in patent: Paragraph 0300; 0301; 0302
[5] Patent: CN103539777, 2016, B. Location in patent: Paragraph 0304; 0305 |
|
|