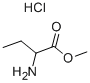
| Identification | Back Directory | [Name]
METHYL D-HOMOALANINATE HCL | [CAS]
85774-09-0 | [Synonyms]
D-2-Abu-OMe HCl
H-D-2-Abu-Ome.HCl
METHYL D-HOMOALANINATE HCL
Methyl (R)-2-aminobutyrate HCL
Methyl (R)-2-aminobutanoate HCl
Methyl D-homoalaninate hydrochloride
(R)-2-AMino-butyric acid Methyl ester
(R)-methyl 2-aminobutanoate hydrochloride
Methyl (R)-2-aminobutanoate hydrochloride
methyl (2R)-2-aminobutanoate hydrochloride
D-2-Amino butanoic acid methyl ester hydrochloride
(R)-2-Aminobutanoic Acid Methyl Ester Hydrochloride
D-alpha-Aminobutyric acid methyl ester hydrochloride
Butanoic acid, 2-aMino-, Methyl ester, hydrochloride , (2R)-
Butanoic acid, 2-aMino-, Methyl ester, hydrochloride (1:1), (2R)- | [EINECS(EC#)]
675-786-7 | [Molecular Formula]
C5H11NO2.HCl | [MDL Number]
MFCD10565758 | [MOL File]
85774-09-0.mol | [Molecular Weight]
153.61 |
| Chemical Properties | Back Directory | [storage temp. ]
Inert atmosphere,Room Temperature | [Appearance]
White to off-white Solid | [Optical Rotation]
Consistent with structure | [InChI]
InChI=1/C5H11NO2.ClH/c1-3-4(6)5(7)8-2;/h4H,3,6H2,1-2H3;1H/t4-;/s3 | [InChIKey]
AHAQQEGUPULIOZ-NDILARRWNA-N | [SMILES]
[C@@H](N)(CC)C(=O)OC.Cl |&1:0,r| |
| Hazard Information | Back Directory | [Uses]
(R)-2-Aminobutanoic Acid Methyl Ester Hydrochloride is used as a reagent in the synthesis of pteridine derivatives which have antibacterial and antifungal activies. (R)-2-Aminobutanoic Acid Methyl Ester Hydrochloride is also used in the synthesis of diazepanones as dipeptidyl peptidase IV inhibitors. | [Synthesis]
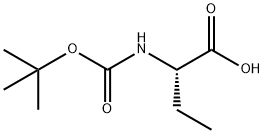
The general procedure for the synthesis of DL-2-aminobutyric acid methyl ester hydrochloride from methanol and Boc-L-2-aminobutyric acid was as follows: 10.0 g (49.2 mmol) of Boc-L-2-aminobutyric acid was dissolved in 100 mL of methanol, and 6 mL (82.2 mmol) of thionyl chloride was added slowly and dropwise at 0 °C. The reaction mixture was stirred at room temperature for 14 h before the solvent was removed by vacuum evaporation. Finally, 7.6 g (101% yield) of DL-2-aminobutyric acid methyl ester hydrochloride was obtained, whose mass spectrometry analysis showed that the (M + H)+ peak was located at 118 m/z. | [References]
[1] Patent: US2010/144681, 2010, A1. Location in patent: Page/Page column 67 |
|
|