| Identification | Back Directory | [Name]
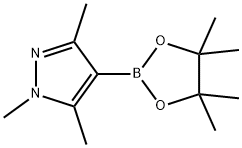
1,3,5-TRIMETHYL-4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-1H-PYRAZOLE | [CAS]
844891-04-9 | [Synonyms]
1,3,5-Trimethylpyrazole-4-boronic Acid Pinacol Ester
1,3,5-TRIMETHYL-1H-PYRAZOLE-4-BORONIC ACID PINACOL ESTER
1,3,5-Trimethyl-1H-pyrazole-4-boronicacid,pinacolester97%
1,3,5-Trimethyl-1H-pyrazole-4-boronic acid pinacol ester 97%
1,3,5-triMethyl-4-(tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
2-(1,3,5-Trimethylpyrazol-4-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
1,3,5-Trimethyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole
1,3,5-TRIMETHYL-4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-1H-PYRAZOLE | [Molecular Formula]
C12H21BN2O2 | [MDL Number]
MFCD06659062 | [MOL File]
844891-04-9.mol | [Molecular Weight]
236.12 |
| Chemical Properties | Back Directory | [Melting point ]
91-93°C | [Boiling point ]
335.7±42.0 °C(Predicted) | [density ]
1.04±0.1 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,Store in freezer, under -20°C | [solubility ]
soluble in Methanol | [form ]
powder to crystal | [pka]
3.13±0.10(Predicted) | [color ]
White to Almost white | [InChIKey]
IZNGYNMIIVJWSO-UHFFFAOYSA-N | [CAS DataBase Reference]
844891-04-9 |
| Hazard Information | Back Directory | [Synthesis]
General procedure for the synthesis of 1,3,5-trimethyl-1H-pyrazole-4-boronic acid pinacol ester from 3,5-dimethylpyrazole-4-boronic acid pinacol ester and iodomethane: 3,5-dimethyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (500 mg, 2.25 mmol) was suspended in acetone (5 mL) in acetone (5 mL), potassium carbonate (622 mg, 4.5 mmol) and iodomethane (0.21 mL, 3.37 mmol) were added. The reaction mixture was heated to 60 °C and kept for 4.5 hours. After completion of the reaction, it was cooled to room temperature, water was added, the aqueous phase was separated and extracted three times with ethyl acetate (EtOAc). The organic phases were combined, washed with water, dried over anhydrous magnesium sulfate (MgSO4), and concentrated in vacuo to afford 1,3,5-trimethyl-1H-pyrazole-4-boronic acid pinacol ester (403 mg, 76%) as a light yellow oil.LCMS (ES+) m/z 237 (M+H)+, retention time (RT) 3.35 min (Method 1). | [References]
[1] Patent: WO2009/71895, 2009, A1. Location in patent: Page/Page column 117
[2] Patent: US2016/176882, 2016, A1. Location in patent: Paragraph 0366; 0367
[3] Patent: WO2017/221100, 2017, A1. Location in patent: Paragraph 00174
[4] Patent: WO2013/96153, 2013, A1. Location in patent: Page/Page column 173-174 |
|
|