| Identification | Back Directory | [Name]
3-Bromo-N-methyl-N-boc-propylamine | [CAS]
828272-19-1 | [Synonyms]
-N-boc-propyL
3-Bromo-N-methyL
N-Boc-N-methyl-3-bromopropylamine
3-Bromo-N-methyl-N-boc-propylamine
tert-Butyl (3-bromopropyl)(methyl)carbamate
tert-butyl N-(3-bromopropyl)-N-methylcarbamate
(3-Bromo-propyl)-methyl-carbamic acid tert-butyl ester
Carbamic acid, (3-bromopropyl)methyl-, 1,1-dimethylethyl ester
N-(3-BroMopropyl)-N-MethylcarbaMic Acid 1,1-DiMethylethyl Ester
Carbamic acid, N-(3-bromopropyl)-N-methyl-, 1,1-dimethylethyl ester | [Molecular Formula]
C9H18BrNO2 | [MOL File]
828272-19-1.mol | [Molecular Weight]
252.15 |
| Chemical Properties | Back Directory | [Boiling point ]
268.4±19.0 °C(Predicted) | [density ]
1.254±0.06 g/cm3(Predicted) | [solubility ]
Chloroform, Dichloromethane, Ethyl Acetate | [pka]
-1.39±0.70(Predicted) | [InChI]
InChI=1S/C9H18BrNO2/c1-9(2,3)13-8(12)11(4)7-5-6-10/h5-7H2,1-4H3 | [InChIKey]
PIUWBRCCWDAPFG-UHFFFAOYSA-N | [SMILES]
C(OC(C)(C)C)(=O)N(CCCBr)C |
| Hazard Information | Back Directory | [Synthesis]
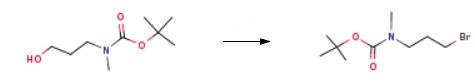
Into a 100 mL round-bottom flask were placed tert-butyl (3- hydroxypropyl)(methyl)carbamate (2.00 g, 10.60 mmol, 1.00 equiv), DCM (20.00 mL) and PPh3 (3.67 g, 13.80 mmol, 1.30 equiv). This was followed by addition of CBr4 (5.20 g, 13.80 mmol, 1.30 equiv) at 0 °C. The resulting solution was stirred for 1.5 h at 0 °C. The reaction mixture was quenched with H2O (50.00 mL), extracted with DCM (100 mL*2). The combined organic phase was washed with Na2CO3 (aq), brine and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column with petroleum ether/ethyl acetate (93%/7%). This resulted in 2.6 g of 3-Bromo-N-methyl-N-boc-propylamine as light yellow oil.
 |
|
|