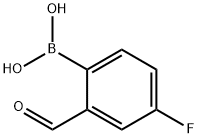
| Identification | Back Directory | [Name]
(4-FLUORO-2-FORMYLPHENYL)BORONIC ACID | [CAS]
825644-26-6 | [Synonyms]
4-Fluoro-2-formylbenzeneboronic acid
Boronic acid, (4-fluoro-2-formylphenyl)-
4-Fluoro-2-formylphenylboronic acid (~90%)
Boronic acid, B-(4-fluoro-2-formylphenyl)-
4-Fluoro-2-formylphenylboronic Acid (contains varying amounts of Anhydride) | [EINECS(EC#)]
200-258-5 | [Molecular Formula]
C7H6BFO3 | [MDL Number]
MFCD10697426 | [MOL File]
825644-26-6.mol | [Molecular Weight]
167.93 |
| Chemical Properties | Back Directory | [Melting point ]
123-125 °C | [Boiling point ]
341.9±52.0 °C(Predicted) | [density ]
1.33±0.1 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [solubility ]
Acetonitrile (Slightly), DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
8.32±0.53(Predicted) | [color ]
White to Pale Yellow |
| Hazard Information | Back Directory | [Uses]
suzuki reaction | [Synthesis]
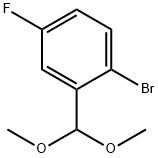
1. Dissolve 2-bromo-5-fluorobenzaldehyde (20.0 g, 98.5 mmol) in 40 mL of methanol. 0.5 mL of concentrated sulfuric acid was added and 13.6 g (128 mmol) of trimethyl orthoformate was added slowly and dropwise. The reaction mixture was refluxed for 1 hour and subsequently cooled to room temperature. The pH was adjusted to 11 with a methanolic solution of concentrated sodium methanolate.After distillation to remove volatiles, the product was purified by vacuum distillation to afford 23.8 g of 1-bromo-2-(dimethoxymethyl)-4-fluorobenzene as a colorless liquid (96.9% yield).1H NMR (CDCl3, 500 MHz) data: δ 7.51 (m, 1H), 7.35 (m, 1H), 6.93 ( m, 1H), 5.50 (s, 1H), 3.38 (s, 6H).
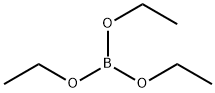
2. 23.8 g (95.0 mmol) of the above product was dissolved in 200 mL of anhydrous ether and 40 mL of tetrahydrofuran under argon protection. The solution was cooled to -75°C using a dry ice/acetone bath. 2.5 M hexane solution of n-butyllithium (42.0 mL, 110.0 mmol) was slowly added dropwise, keeping the reaction temperature below -70°C. The reaction temperature was kept below -70°C. The reaction temperature was kept below -70°C. After stirring for 1 h, 16.1 g (110.0 mmol) of triethyl borate was added slowly and the temperature continued to be maintained below -70°C. The reaction temperature was kept below -70°C. The reaction temperature was kept below -70°C. The cooling bath was removed and the pH was adjusted to 3 with 3 M aqueous hydrochloric acid while allowing the temperature to rise to 5°C. The aqueous layer was separated and extracted with ether (2 x 50 mL). The organic layers were combined and the solvent was partially removed under vacuum. Distillation was continued by adding water. After cooling, the precipitated solid was filtered and dried in air to give 14.3 g of 4-fluoro-2-formylphenylboronic acid (89.5% yield).11B NMR (64 MHz, acetone-d6) data: δ 30.9 ppm.
3. Compounds 1, 3 and 4 were synthesized by a similar method to the corresponding fluorosubstituted 2-bromobenzaldehydes. The overall (two-step) yields were 1 (85.4%), 3 (78.3%), and 4 (92.1%). | [References]
[1] Journal of Fluorine Chemistry, 2016, vol. 187, p. 1 - 8 |
|
|