| Identification | Back Directory | [Name]
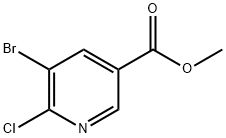
Methyl 5-bromo-6-chloropyridine-3-carboxylate | [CAS]
78686-77-8 | [Synonyms]
Methyl 5-bromo-6-chl
METHYL 5-BROMO-6-CHLORONICOTINATE
Methyl-5-bromo-6-chloronicotinic acid
5-BROMO-6-CHLORONICOTINIC ACID METHYL ESTER
methyl 5-bromo-6-chloropyridine-3-carboxylate
Methyl 3-bromo-2-chloropyridine-5-carboxylate
3-Pyridinecarboxylic acid, 5-bromo-6-chloro-, methyl ester
Methyl 5-bromo-6-chloropyridine-3-carboxylate ISO 9001:2015 REACH
Methyl 5-bromo-6-chloropyridine-3-carboxylate, Methyl 3-bromo-2-chloropyridine-5-carboxylate | [EINECS(EC#)]
-0 | [Molecular Formula]
C7H5BrClNO2 | [MDL Number]
MFCD03844855 | [MOL File]
78686-77-8.mol | [Molecular Weight]
250.48 |
| Chemical Properties | Back Directory | [Melting point ]
75-77°C | [Boiling point ]
279.2±35.0 °C(Predicted) | [density ]
1.684 | [refractive index ]
1.568 | [storage temp. ]
Inert atmosphere,2-8°C | [solubility ]
Chloroform (Slightly), Methanol (Slightly), Water (Slightly) | [form ]
Solid | [pka]
-2.84±0.10(Predicted) | [color ]
White to Off-White | [InChI]
InChI=1S/C7H5BrClNO2/c1-12-7(11)4-2-5(8)6(9)10-3-4/h2-3H,1H3 | [InChIKey]
WINGWVOUOFMOJQ-UHFFFAOYSA-N | [SMILES]
C1=NC(Cl)=C(Br)C=C1C(OC)=O | [CAS DataBase Reference]
78686-77-8 |
| Hazard Information | Back Directory | [Uses]
Methyl 5-Bromo-6-chloronicotinate can be used to treat diseases related to S1PR1. | [Synthesis]
Step 1: Synthesis of methyl 5-bromo-6-chloronicotinate (61). A mixture of 5-bromo-6-chloronicotinic acid (60) (0.2 g, 0.84 mmol), potassium carbonate (K2CO3) (0.3 g, 2.1 mmol) and iodomethane (0.178 g, 1.2 mmol) in N,N-dimethylformamide (DMF) (10 mL) was stirred for 16 h at 0 °C and subsequently raised to room temperature. Upon completion of the reaction, the reaction mixture was diluted with water (20 mL) and extracted with ethyl acetate (EtOAc) (2 x 20 mL). The organic phases were combined and washed sequentially with saturated aqueous sodium bicarbonate (NaHCO3) (20 mL) and brine (2 x 20 mL), dried over anhydrous sodium sulfate (Na2SO4) and concentrated to afford the target product methyl 5-bromo-6-chloronicotinate (61) (0.2 g, 94.7% yield). | [References]
[1] Patent: WO2017/4608, 2017, A1. Location in patent: Paragraph 0183 |
|
|