| Identification | Back Directory | [Name]
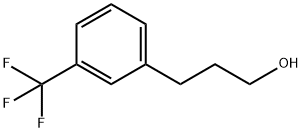
3-(3'-TRIFLUOROMETHYL PHENYL) PROPANOL | [CAS]
78573-45-2 | [Synonyms]
Cinacalcet I
Cinacalcet InterMediates
Cinacalcet Intermediate 1
3-(Trifluoromethyl)benzenepropanol
Benzenepropanol,3-(trifluoroMethyl)-
3-(3'-TRIFLUOROMETHYL PHENYL) PROPANOL
3-(3-TRIFLUOROMETHYL-PHENYL)-PROPAN-1-OL
3-(3-(trifluoroMrthyl)pheny1)propan-1-ol
3-[3-(Trifluoromethyl)phenyl]-1-propanol
3-(3-Hydroxyprop-1-yl)benzotrifluoride, 3-(3-Hydroxyprop-1-yl)-alpha,alpha,alpha-trifluorotoluene | [EINECS(EC#)]
616-635-7 | [Molecular Formula]
C10H11F3O | [MDL Number]
MFCD08706408 | [MOL File]
78573-45-2.mol | [Molecular Weight]
204.189 |
| Chemical Properties | Back Directory | [Appearance]
Brown Oil | [Boiling point ]
68-71℃/0.4mm | [density ]
1.10 | [refractive index ]
1.4620 to 1.4660 | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Liquid | [pka]
15.04±0.10(Predicted) | [color ]
Colorless | [InChI]
InChI=1S/C10H11F3O/c11-10(12,13)9-5-1-3-8(7-9)4-2-6-14/h1,3,5,7,14H,2,4,6H2 | [InChIKey]
QWXKQVIMGVVIBX-UHFFFAOYSA-N | [SMILES]
C1(CCCO)=CC=CC(C(F)(F)F)=C1 |
| Hazard Information | Back Directory | [Chemical Properties]
Brown Oil | [Uses]
3-(Trifluoromethyl)benzenepropanol-d4 is an intermediate in the synthesis of Cinacalcet-d4 Hydrochloride (C441803), a labeled analogue of Cinacalcet Hydrochloride (C441800), the (R) enantiomer of Cinacalcet which is used in clinical trial in secondary hyperparathyroidism. | [Definition]
ChEBI: 3-[3-(trifluoromethyl)phenyl]propan-1-ol is an organofluorine compound that is 3-phenylpropan-1-ol bearing a trifluoromethyl group at position 3 on the phenyl ring. It is an organofluorine compound and a member of propan-1-ols. | [Synthesis]
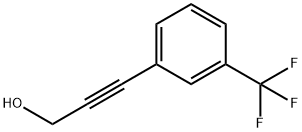
To a solution of 14.5 g (72.5 mmol) of purified 3-(3-trifluoromethylphenyl)propargyl alcohol in 50 mL of 2-propanol was added 0.38 g of 10% Pd/C (Selcat Q6). The reaction mixture was subjected to hydrogenation at a temperature of 42-45°C and a pressure of 5 bar until all feedstocks were completely converted (about 5 hours). Upon completion of the reaction, it was filtered to remove the catalyst and the catalyst was washed with a small amount of 2-propanol. Subsequently, the filtrate was concentrated by evaporation under reduced pressure. The crude product obtained (14.5 g, 100.0%) was purified by distillation under reduced pressure, resulting in 10.5 g (yield: 72.1%) of the target product 3-(3-trifluoromethylphenyl)propanol as an almost colorless oil (boiling point: 58-60 °C/1.1-1.5 mbar). | [References]
[1] Organic Letters, 2019, vol. 21, # 1, p. 65 - 69
[2] Patent: US2010/267988, 2010, A1. Location in patent: Page/Page column 4
[3] Patent: EP2327684, 2011, A1. Location in patent: Page/Page column 6
[4] Patent: US2011/124917, 2011, A1. Location in patent: Page/Page column 3 |
|
|