| Identification | Back Directory | [Name]
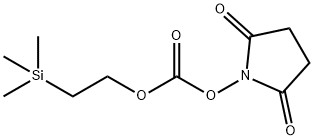
1-(2-(TRIMETHYLSILYL)ETHOXYCARBONYLOXY)& | [CAS]
78269-85-9 | [Synonyms]
Teoc-OSu
1-(2-(TRIMETHYLSILYL)ETHOXYCARBONYLOXY)&
(TriMethylsilyl)ethoxycarbonyloxy]succiniMide
1-[[[2-(Trimethylsilyl)ethoxy]carbonyl]oxy]-2
N-[2-(Trimethylsilyl)ethoxycarbonyloxy]succinimide
(triMethylsilyl)ethoxy]carbonyl}oxy)pyrrolidine-2,5-dione
N-[2-(Trimethylsilyl)ethoxycarbonyloxy]succinimide≥ 98%(N)
TEOC-OSU N-[2-(trimethylsilyl)ethoxycarbonyloxy]succinimide
N-[2-(trimethylsilyl)ethoxycarbonyloxy]succinimide(Teoc-Osu)
2,5-Dioxopyrrolidin-1-yl (2-(trimethylsilyl)-ethyl) carbonate
1-[[[2-(Trimethylsilyl)ethoxy]carbonyl]oxy]-2,5-pyrrolidinedione
1-({[2-(trimethylsilyl)ethoxy]carbonyl}oxy)pyrrolidine-2,5-dione
Carbonic acid,2,5-dioxo-1-pyrrolidinyl [2-(triMethylsilyl)ethyl] ester
Carbonic acid 2,5-dioxo-pyrrolidin-1-yl [2-(trimethylsilyl)ethyl] ester | [Molecular Formula]
C10H17NO5Si | [MDL Number]
MFCD02683467 | [MOL File]
78269-85-9.mol | [Molecular Weight]
259.33 |
| Chemical Properties | Back Directory | [Melting point ]
83-88 °C(lit.)
| [Boiling point ]
308.6±44.0 °C(Predicted) | [density ]
1.17±0.1 g/cm3(Predicted) | [storage temp. ]
2-8°C | [solubility ]
soluble in Methanol | [form ]
powder to crystal | [color ]
White to Almost white | [Hydrolytic Sensitivity]
7: reacts slowly with moisture/water | [InChI]
InChI=1S/C10H17NO5Si/c1-17(2,3)7-6-15-10(14)16-11-8(12)4-5-9(11)13/h4-7H2,1-3H3 | [InChIKey]
FLDNDAMSCINJDX-UHFFFAOYSA-N | [SMILES]
C(OCC[Si](C)(C)C)(=O)ON1C(=O)CCC1=O |
| Hazard Information | Back Directory | [Chemical Properties]
White to off-white powder | [Uses]
1-[2-(Trimethylsilyl)ethoxycarbonyloxy]pyrrolidin-2,5-dione (Teoc-OSu) may be used as an acylating reagent to produce Teoc-amino acid derivatives. | [General Description]
1-[2-(Trimethylsilyl)ethoxycarbonyloxy]pyrrolidin-2,5-dione (Teoc-OSu) is a silicon-based reagent that can be prepared from 2-trimethylsilylethyl carbonochloridite and N-hydroxysuccinimide. | [Synthesis]
2-(Trimethylsilyl)ethyl chloroformate (S13) was dissolved in acetonitrile (105 mL) at 0 °C. Subsequently, N-hydroxybutanediimide (5.22 g, 45.4 mmol, 1.30 eq.) and triethylamine (4.59 g, 45.4 mmol, 1.30 eq.; dissolved in 11.0 mL acetonitrile) were added to the solution. The reaction mixture was kept stirred at 0 °C for 16 hours. After completion of the reaction, the mixture was poured into water. The aqueous layer was extracted with ether (6 times). The organic layers were combined and washed sequentially with water (2 times), 1.0 M hydrochloric acid and then with water, followed by drying with magnesium sulfate. The dried organic phase was filtered and concentrated under reduced pressure to give 1-[2-(trimethylmethylsilyl)ethoxycarbonyloxy]pyrrolidine-2,5-dione (S14, 7.72 g, 29.8 mmol, 85% yield in two steps) as a colorless solid. | [References]
[1] Beilstein Journal of Organic Chemistry, 2016, vol. 12, p. 564 - 570
[2] Synthesis, 1987, # 4, p. 346 - 349
[3] Patent: US6627660, 2003, B1
[4] Patent: US2004/116391, 2004, A1 |
|
|




![(2-[(CHLOROCARBONYL)OXY]ETHYL)TRIMETHYLSILANE](/CAS/20180601/GIF/20160-60-5.gif)
