| Identification | Back Directory | [Name]
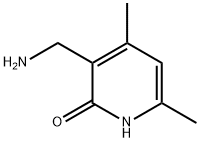
2(1H)-Pyridinone, 3-(aminomethyl)-4,6-dimethyl- (9CI) | [CAS]
771579-27-2 | [Synonyms]
N-BUTYL-2-CHLOROISONICOTINAMIDE
3-(Aminomethyl)-4,6-dimethyl-2(1H)-pyridinone
3-(aminomethyl)-4,6-dimethyl-1H-pyridin-2-one
2(1H)-Pyridinone,3-(aMinoMethyl)-4,6-diMethyl-
3-(AMinoMethyl)-4,6-diMethyl-1,2-dihydropyridin-2-one
2(1H)-Pyridinone, 3-(aminomethyl)-4,6-dimethyl- (9CI)
3-(AMinoMethyl)-4,6-diMethyl-1,2-dihydropyridine-2-one
3-(Aminomethyl)-4,6-Dimethylpyridin-2(1H)-One(WX603161)
(4,6-dimethyl-2-oxo-1H-pyridin-3-yl)methylazanium chloride
2(1H)-Pyridinone,3-(aMinoMethyl)-4,6-diMethyl-,hydrochloride(1:1)
2(1H)-Pyridinone, 3-(aminomethyl)-4,6-dimethyl- (9CI) ISO 9001:2015 REACH | [Molecular Formula]
C8H12N2O | [MDL Number]
MFCD06213260 | [MOL File]
771579-27-2.mol | [Molecular Weight]
152.19 |
| Chemical Properties | Back Directory | [Boiling point ]
348.1±34.0 °C(Predicted) | [density ]
1.054±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,2-8°C | [pka]
12.40±0.10(Predicted) | [Appearance]
White to yellow Solid |
| Hazard Information | Back Directory | [Uses]
3-(Aminomethyl)-4,6-dimethylpyridin-2(1H)-one is a reactant in the preparation of pyrazolopyridine and indazoles as histone lysine methyltransferase EZH2 inhibitors. | [Synthesis]
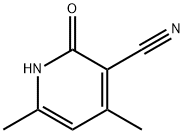
General procedure for the synthesis of 3-(aminomethyl)-4,6-dimethylpyridin-2(1H)-one from 2-oxo-4,6-dimethyl-1,2-dihydropyridine-3-carbonitrile: Carbon-loaded palladium (10%, 324 mg) and platinum oxide (21.8 mg) were added to 4,6-dimethyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (3 g, 20.3 mmol) to a mixture of sodium acetate (3.1 g, 37.5 mmol) and acetic acid (100 mL). The reaction mixture was stirred under hydrogen atmosphere for 48 hours. Upon completion of the reaction, the reaction mixture was filtered through a diatomaceous earth pad and the filtrate was concentrated to dryness. The residue was treated with concentrated hydrochloric acid (3 mL) and ethanol (15 mL), cooled to 0°C and stirred at that temperature for 2 hours to form a suspension. The precipitate was collected by filtration, washed sequentially with cold ethanol and ether, and dried under vacuum to give a white solid product (3.5 g, 92% yield) with a melting point of 253-258 °C. The structure of the product was confirmed by 1H NMR (400 MHz, DMSO-d6) and 13C NMR (100 MHz, DMSO-d6), and analyzed by high-resolution mass spectrometry (HRMS-ESI), and the results were in agreement with expectations. | [References]
[1] Synthetic Communications, 2016, vol. 46, # 14, p. 1215 - 1222
[2] Patent: WO2013/75083, 2013, A1. Location in patent: Paragraph 00177; 00178
[3] Patent: US9206128, 2015, B2. Location in patent: Page/Page column 103; 104
[4] ACS Medicinal Chemistry Letters, 2012, vol. 3, # 12, p. 1091 - 1096
[5] Journal of Medicinal Chemistry, 2016, vol. 59, # 16, p. 7617 - 7633 |
|
|