| Identification | Back Directory | [Name]
2-Propynoic acid, 3-(4-chlorophenyl)-, methyl ester | [CAS]
7515-18-6 | [Synonyms]
Methyl 3-(4-Chlorophenyl)propiolate
Methyl (4-Chlorophenyl)propargylate
3-(4-Chlorophenyl)propiolic Acid Methyl Ester
(4-Chlorophenyl)propargylic Acid Methyl Ester
(4-Chloro-phenyl)-propynoic acid methyl ester
3-(4-Chlorophenyl)-2-propynoic Acid Methyl Ester
2-Propynoic acid, 3-(4-chlorophenyl)-, methyl ester | [Molecular Formula]
C10H7ClO2 | [MDL Number]
MFCD01830788 | [MOL File]
7515-18-6.mol | [Molecular Weight]
194.61 |
| Chemical Properties | Back Directory | [Melting point ]
95 °C | [Boiling point ]
277.6±23.0 °C(Predicted) | [density ]
1.26±0.1 g/cm3(Predicted) | [solubility ]
soluble in Acetone | [form ]
powder to crystal | [color ]
White to Light yellow to Light orange |
| Hazard Information | Back Directory | [Description]
2-Propynoic acid, 3-(4-chlorophenyl)-, methyl ester is a useful research compound. Its molecular formula is C10H7ClO2 and its molecular weight is 194.61 g/mol. | [Uses]
2-Propynoic acid, 3-(4-chlorophenyl)-, methyl ester, also known as Methyl 3-(4-Chlorophenyl)propiolate, is an organic compound that has a wide range of applications in scientific research. | [Biological Activity]
The biological activity of 2-Propynoic acid, 3-(4-chlorophenyl)-, methyl ester is attributed to its ability to inhibit the activity of histone deacetylases (HDACs) which are enzymes that play a crucial role in the regulation of gene expression. This ability leads to the accumulation of acetylated histones, which results in the activation of tumor suppressor genes and the inhibition of oncogenes. | [Synthesis]
The synthesis of 2-Propynoic acid, 3-(4-chlorophenyl)-, methyl ester is as follows:
Into a 10 mL reaction tube was added 212 mg methyl-3-(4-chlorophenyl)-3-oxopropionic acid (1.0 mmol), 608 mg DBU (4.0 mmol), 7.5 mL DMSO, and a balloon filled with sulfuryl fluoride gas for a long time. In the form of a needle, air is bubbling below the liquid surface. The reaction system was reacted at room temperature for 3 hours. The progress of the reaction was detected by a thin layer chromatography plate. After the reaction was completed, the rotary evaporator was used to spin dry, and the residue was purified by silica gel column chromatography. The eluent was petroleum ether:ethyl acetate=10:1, to obtain methyl- 3-(4-Chlorophenyl)propynoic acid (165 mg, 85%).
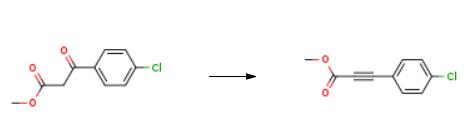 |
|
| Company Name: |
TCI Europe
|
| Tel: |
320-37350700 |
| Website: |
https://www.tcichemicals.com/de/de/index.html |
| Company Name: |
TCI AMERICA
|
| Tel: |
800-4238616 |
| Website: |
https://www.tcichemicals.com/en/us/index.html |
| Company Name: |
TCI Chemicals
|
| Tel: |
021-67121386, 800-988-0390 |
| Website: |
www.tcichemicals.com |
| Company Name: |
Energy Chemical
|
| Tel: |
021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|