| Identification | Back Directory | [Name]
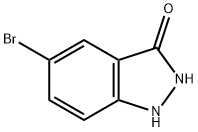
5-BROMO-3-HYDROXY (1H)INDAZOLE | [CAS]
7364-27-4 | [Synonyms]
5-Bromo-1H-indazol-3-ol
5-Bromoindazol-3(2H)-one
5-BROMO-3-HYDROXYINDAZOLE
5-BROMO-3-HYDROXY (1H)INDAZOLE
5-BROMO-1,2-DIHYDRO-INDAZOL-3-ONE
5-broMo-1,2-dihydro-3H-Indazol-3-one
3H-Indazol-3-one, 5-broMo-1,2-dihydro- | [Molecular Formula]
C7H5BrN2O | [MDL Number]
MFCD10697926 | [MOL File]
7364-27-4.mol | [Molecular Weight]
213.03 |
| Chemical Properties | Back Directory | [Boiling point ]
268.4±19.0 °C(Predicted) | [density ]
1.728±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [form ]
solid | [pka]
11.02±0.20(Predicted) | [Appearance]
Light brown to brown Solid |
| Hazard Information | Back Directory | [Chemical Properties]
White to yellow to gray to brown Solid
| [Uses]
5-BROMO-3-HYDROXY (1H)INDAZOLE is a potential pharmaceutical intermediate, commonly used in the preparation of various drugs and biologically active compounds.
| [Synthesis]
Part B: Methyl 5-bromo-2-fluorobenzoate (1.02 g, 4.2 mmol) was dissolved in 8 mL of ethanol and hydrazine monohydrate (4.0 mL, 80 mmol) was added. The reaction mixture was placed in a Biotage microwave reactor and stirred at 100 °C for 30 min. The reaction was found to be incomplete by LCMS, so the mixture was concentrated and fresh ethanol (6 mL) and hydrazine monohydrate (6 mL) were added. The reaction mixture was again placed in a microwave reactor and stirred at 100 °C for 30 min. Upon completion of the reaction, the mixture was concentrated to dryness to afford the target product 5-bromo-1H-indazol-3-ol (801 mg, 89% yield). HPLC-MS analysis results: t R = 1.21 min (UV 254 nm); mass calculated value C7H5BrN2O 212.0/214.0, measured value LCMS m/z 213.1/215.1 (M + H ). | [References]
[1] Patent: WO2010/54279, 2010, A1. Location in patent: Page/Page column 109-110
[2] Patent: WO2017/46318, 2017, A1. Location in patent: Page/Page column 54; 55
[3] Patent: WO2015/150565, 2015, A1. Location in patent: Page/Page column 75; 76 |
|
|