| Identification | Back Directory | [Name]
1,3-Dimethyl-1H-benzo[d]imidazol-3-ium iodide | [CAS]
7181-87-5 | [Synonyms]
1,3-dimethylbenzoimidazole
1,3-Dimethylbenzimidazolium iodide
N,N'-Dimethylbenzimidazolium iodide
1,3-Dimethyl-1H-benzimidazolium iodide
1,3-Dimethyl-1H-benzimidazol-3-ium iodide
1,3-Dimethyl-1H-benzo[d]imidazol-3-ium iodide | [Molecular Formula]
C9H11IN2 | [MDL Number]
MFCD00491277 | [MOL File]
7181-87-5.mol | [Molecular Weight]
274 |
| Chemical Properties | Back Directory | [Melting point ]
137-142°C | [storage temp. ]
under inert gas (nitrogen or Argon) at 2–8 °C | [form ]
solid | [Appearance]
Off-white to light brown Solid | [InChI]
InChI=1S/C9H11N2.HI/c1-10-7-11(2)9-6-4-3-5-8(9)10;/h3-7H,1-2H3;1H/q+1;/p-1 | [InChIKey]
RQGURHMTNSNBQX-UHFFFAOYSA-M | [SMILES]
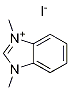
C1=[N+](C)C2=CC=CC=C2N1C.[I-] |
| Hazard Information | Back Directory | [Uses]
Catalyst for:
- Domino ring-opening redox amidation Knoevenagel condensation
- Intramolecular stereoselective protonation
- Grignard allylic substitution
- Acylation of alcohols
- Umpolung reactions
| [reaction suitability]
reagent type: catalyst | [Synthesis]
The general procedure for the synthesis of 1,3-dimethyl-1H-benzo[d]imidazol-3-ium iodide from benzimidazole and iodomethane was as follows: 1H-benzo[d]imidazole (1 g, 8.5 mmol), K2CO3 (1.18 g, 8.5 mmol), iodomethane (2.1 mL, 33 mmol), and acetonitrile (15 mL) were added sequentially to a 25 mL Schlenk tube. The reaction tube was sealed, evacuated and filled with nitrogen three times to displace the air. The mixture was placed in a preheated 83°C oil bath, stirred and heated for 18 hours. Upon completion of the reaction, the reaction mixture was rapidly filtered while hot to remove insoluble material (immediate precipitation of white product was observed). The filtrate was quickly transferred to an ice water bath for cooling to induce the precipitation of white crystals. The crystals were collected by filtration, washed with petroleum ether (PE) and finally dried under reduced pressure to afford the target compound 1,3-dimethyl-1H-benzo[d]imidazol-3-ium iodide (NHC precursor J, 1.3 g, 56% yield). | [References]
[1] Journal of Medicinal Chemistry, 2010, vol. 53, # 24, p. 8608 - 8618
[2] Organometallics, 2013, vol. 32, # 6, p. 2033 - 2036
[3] Journal of Heterocyclic Chemistry, 2017, vol. 54, # 6, p. 3646 - 3655
[4] Dalton Transactions, 2014, vol. 43, # 16, p. 5978 - 5982
[5] Organic Letters, 2007, vol. 9, # 17, p. 3437 - 3439 |
|
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
| Company Name: |
Rhawn Reagent
|
| Tel: |
400-400-1332688 18019345275 |
| Website: |
http://www.rhawn.cn |
|