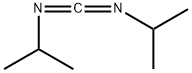| Identification | Back Directory | [Name]
2-TERT-BUTYL-1,3-DIISOPROPYLISOUREA | [CAS]
71432-55-8 | [Synonyms]
2-TERT-BUTYL-1
3-DIISOPROPYLISOUREA
2-TERT-BUTYL-1,3-DIISOPROPYLISOUREA
O-tert-Butyl-N,N'-diisopropylisourea
tert-Butyl N,N'-diisopropylcarbaMiMidate
O-tert-Butyl-N,N'-diisopropylisourea >
tert-Butyl N,N'-diisopropylimidocarbamate
tert-butyl N,N'-bis(1-methylethyl)imidocarbamate
CarbaMiMidic acid, N,N'-bis(1-Methylethyl)-, 1,1-diMethylethyl ester | [Molecular Formula]
C11H24N2O | [MDL Number]
MFCD06657672 | [MOL File]
71432-55-8.mol | [Molecular Weight]
200.32 |
| Chemical Properties | Back Directory | [Boiling point ]
61°C/10mmHg(lit.) | [density ]
0.89±0.1 g/cm3(Predicted) | [refractive index ]
1.4240-1.4280 | [storage temp. ]
0-10°C | [form ]
clear liquid | [pka]
9.54±0.50(Predicted) | [color ]
Colorless to Almost colorless | [InChI]
InChI=1S/C11H24N2O/c1-8(2)12-10(13-9(3)4)14-11(5,6)7/h8-9H,1-7H3,(H,12,13) | [InChIKey]
FESDUDPSRMWIDL-UHFFFAOYSA-N | [SMILES]
C(=NC(C)C)(OC(C)(C)C)NC(C)C |
| Hazard Information | Back Directory | [Uses]
2-tert-Butyl-1,3-diisopropylisourea is a useful synthetic intermediate in the synthesis of 5-Oxo Atorvastatin tert-Butyl Ester (O847160) which is a Boc-protected derivative of Atorvastatin. 2-tert-Butyl-1,3-diisopropylisourea is also used as a reagent in the total synthesis of Citrafungin A which is an antifungal natural product. | [Synthesis]
GENERAL STEPS: CuCl (63.4 mg, 0.64 mmol, 1 mol%) was added to a solution of N,N'-diisopropylcarbodiimide (10.0 mL, 63.9 mmol, 1.0 equiv) in tert-butanol (6.97 mL, 73.5 mmol, 1.15 equiv). The reaction mixture was stirred at room temperature for 14 hours. After completion of the reaction, purification was carried out by distillation under reduced pressure (80°C, 25 mmHg). 2-tert-butyl-1,3-diisopropylisourea was obtained as a colorless oil (10.4 g, 51.9 mmol, 81% yield). The product was characterized by 1H NMR (300 MHz, CDCl3): δ 3.80-3.58 (1H, m, CH), 3.31-2.97 (1H, m, CH), 1.44 (9H, s, tBu), 1.19-1.01 (12H, m, Me2×2). | [References]
[1] Organic Letters, 2005, vol. 7, # 13, p. 2615 - 2618
[2] Chemistry - A European Journal, 2007, vol. 13, # 30, p. 8543 - 8563
[3] Beilstein Journal of Organic Chemistry, 2011, vol. 7, p. 1486 - 1493
[4] Journal of Medicinal Chemistry, 2015, vol. 58, # 10, p. 4204 - 4219
[5] Patent: EP2952517, 2015, A1. Location in patent: Paragraph 0217 |
|
|