| Identification | Back Directory | [Name]
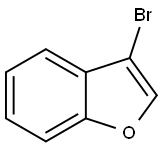
3-BROMO-1-BENZOFURAN | [CAS]
59214-70-9 | [Synonyms]
3-BroMobenzofuran
Benzofuran, 3-broMo-
bromo-3 benzofuranne
3-BROMO-1-BENZOFURAN
3-Bromobenzo[b]furan
3-Bromo-1-benzofurane
3-Bromobenzo[b]furan 97%
3-Bromo-1-benzofuran ,97%
3-Bromo-1-Benzofuran,>95%
3-bromo-1-benzofuran USP/EP/BP | [Molecular Formula]
C8H5BrO | [MDL Number]
MFCD01029425 | [MOL File]
59214-70-9.mol | [Molecular Weight]
197.03 |
| Chemical Properties | Back Directory | [Melting point ]
35 °C | [Boiling point ]
110 | [density ]
1.608±0.06 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,Store in freezer, under -20°C | [form ]
solid | [color ]
Yellow | [InChI]
InChI=1S/C8H5BrO/c9-7-5-10-8-4-2-1-3-6(7)8/h1-5H | [InChIKey]
ICJNAOJPUTYWNV-UHFFFAOYSA-N | [SMILES]
O1C2=CC=CC=C2C(Br)=C1 |
| Hazard Information | Back Directory | [Chemical Properties]
White solid | [Synthesis]
The general procedure for the synthesis of 3-bromo-1-benzofuran from the compound (CAS: 109210-15-3) was as follows: first, potassium hydroxide pellets (9.3 g, 165 mmol) were dissolved in ethanol (40 mL) and the solution was cooled to 0 °C. Subsequently, 2,3-dibromo-2,3-dihydrobenzo[b]furan (23.0 g, 82.7 mmol) was dissolved in pre-cooled ethanol (90 mL) and the solution was added dropwise to the ethanol solution of potassium hydroxide at a constant temperature of 0 °C. After the dropwise addition, the reaction mixture was heated to reflux for 2 hours. After completion of the reaction, the mixture was concentrated under vacuum and then diluted by adding water (100 mL). The aqueous layer was extracted with ethyl acetate (3 × 100 mL). All organic phases were combined and washed with brine (100 mL), followed by drying with anhydrous magnesium sulfate and final concentration to give 14.7 g (90% yield) of the target product 3-bromo-1-benzofuran as an oil. | [References]
[1] Patent: US2006/287386, 2006, A1. Location in patent: Page/Page column 17
[2] Chemistry - A European Journal, 2015, vol. 21, # 7, p. 3115 - 3128 |
|
|




![2,3-dibromo-2,3-dihydro-benzo[b]furan](/CAS/20180703/GIF/109210-15-3.gif)
