| Identification | Back Directory | [Name]
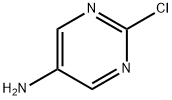
5-Amino-2-chloropyrimidine | [CAS]
56621-90-0 | [Synonyms]
2-CHLOROPYRIMIDIN-5-AMINE
5-AMINO-2-CHLOROPYRIMIDINE
5-amine-2-Chloropyrimidine
5-PYRIMIDINAMINE, 2-CHLORO-
2-Chloro-pyrimidin-5-ylamine
5-AMINO-2-CHLOROPYRIMIDINE,98%
5-Amino-2-chloropyrimidine ,97%
5-AMino-2-chloropyriMidine 97%
5-Pyrimidinamine, 2-chloro- (9CI)
2-Chloropyrimidin-5-amine, 5-Amino-2-chloro-1,3-diazine | [Molecular Formula]
C4H4ClN3 | [MDL Number]
MFCD06412560 | [MOL File]
56621-90-0.mol | [Molecular Weight]
129.55 |
| Chemical Properties | Back Directory | [Melting point ]
198-199℃ | [Boiling point ]
338.8±15.0 °C(Predicted) | [density ]
1.437±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [form ]
Crystalline Powder or Solid | [pka]
-0.08±0.10(Predicted) | [color ]
Yellow to brown | [InChI]
InChI=1S/C4H4ClN3/c5-4-7-1-3(6)2-8-4/h1-2H,6H2 | [InChIKey]
DZBKIOJXVOECRA-UHFFFAOYSA-N | [SMILES]
C1(Cl)=NC=C(N)C=N1 |
| Hazard Information | Back Directory | [Chemical Properties]
Light yellow solid | [Uses]
5-Amino-2-chloropyrimidine is an aminopyrimidine derivative, a fibroblast growth factor receptor 4 inhibitor with anticancer activity. | [Synthesis]
Example 7 Synthesis of 5-amino-2-chloropyrimidine (Compound 40): according to the reaction scheme, 140 g (0.88 mol) of 2-chloro-5-nitropyrimidine (39) was dissolved in 700 mL of ethanol (EtOH) with the addition of 1400 mL of acetic acid, 700 mL of water and 197 g of iron powder (70 μm sieve, <212 μm). The reaction mixture was heated at 70 °C overnight and subsequently cooled to room temperature. After completion of the reaction, filtration was performed. EtOH was removed from the filtrate under vacuum and the pH was adjusted to 8 with 12N NaOH, followed by continuous liquid-liquid extraction with ethyl acetate (EtOAc) overnight to extract the product. The resulting filter cake was washed with EtOAc and the combined EtOAc layers were washed sequentially with water and brine, dried with magnesium sulfate and filtered. The solvent was removed under vacuum and the product was recrystallized with EtOH to give 97.2 g (85% yield) of the final light brown solid product 39.1H NMR (DMSO-d6) δ 8.94 (s, 2H), 5.77 (brs, 2H). | [References]
[1] Tetrahedron, 2014, vol. 70, # 35, p. 5730 - 5738
[2] Patent: WO2017/176812, 2017, A1. Location in patent: Paragraph 0267
[3] Patent: US2014/235858, 2014, A1. Location in patent: Paragraph 0093
[4] Journal of Medicinal Chemistry, 2015, vol. 58, # 22, p. 8796 - 8805
[5] Patent: US2011/9405, 2011, A1. Location in patent: Page/Page column 56 |
|
|