| Identification | Back Directory | [Name]
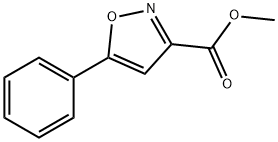
5-PHENYL-ISOXAZOLE-3-CARBOXYLIC ACID METHYL ESTER | [CAS]
51677-09-9 | [Synonyms]
AKOS B024670
AKOS BBS-00002559
ART-CHEM-BB B024670
TIMTEC-BB SBB009684
5-phenylisoxazole-3-carboxylate
3-(Methoxycarbonyl)-5-phenylisoxazole
METHYL 5-PHENYLISOXAZOLE-3-CARBOXYLATE
METHYL 5-PHENYL-3-ISOXAZOLECARBOXYLATE
Methyl 5-phenyl-1,2-oxazole-3-carboxylate
5-Phenyl-3-isoxazolecarboxylic acid methyl ester
5-PHENYL-ISOXAZOLE-3-CARBOXYLIC ACID METHYL ESTER
3-Isoxazolecarboxylic acid, 5-phenyl-, Methyl ester
JR-7047, Methyl 5-phenylisoxazole-3-carboxylate, 97% | [Molecular Formula]
C11H9NO3 | [MDL Number]
MFCD02159553 | [MOL File]
51677-09-9.mol | [Molecular Weight]
203.19 |
| Chemical Properties | Back Directory | [Melting point ]
81°C | [Boiling point ]
370.2±30.0 °C(Predicted) | [density ]
1.204±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [form ]
solid | [pka]
-6.43±0.28(Predicted) | [Appearance]
White to off-white Solid | [InChIKey]
XMPLCJOQBDGMKT-UHFFFAOYSA-N |
| Hazard Information | Back Directory | [Uses]
Methyl 5-Phenylisoxazole-3-carboxylate is a useful reagent for reactions with isoxazoles. | [Synthesis]
GENERAL METHODS: Alkyne oxime ether 1 (0.15 mmol) was dissolved in THF (5 mL) and phenol (28 mg, 0.3 mmol) and AgBF4 (5.8 mg, 0.03 mmol) were added sequentially. The reaction system was equipped with a drying tube (filled with silica gel) and heated to reflux at 50 °C in an oil bath. The reaction process was monitored by TLC with continuous stirring for 1-12 hours. After completion of the reaction, the reaction mixture was concentrated under reduced pressure. The residue was purified by preparative thin layer chromatography (unfolding reagent: hexane/ethyl acetate = 3-10:1) to afford the target product methyl 5-phenylisoxazole-3-carboxylate (3a and 3j). The physical properties and spectral data of the obtained compounds were in agreement with those reported in the literature. | [References]
[1] Tetrahedron, 2011, vol. 67, # 25, p. 4612 - 4615 |
|
|




![3-Butynoic acid, 4-phenyl-2-[(phenylmethoxy)imino]-, methyl ester, (2E)-](/CAS/20210305/GIF/1313396-20-1.gif)
