| Identification | Back Directory | [Name]
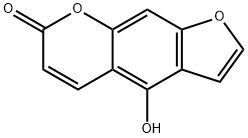
BERGAPTOL | [CAS]
486-60-2 | [Synonyms]
BERGAPTOL
NSC 341958
BERGAPTOL hplc
5-HYDROXYPSORALEN
4-HYDROXYPSORALEN
4-Hydroxybergapten
BERGAPTOL WITH HPLC
5-Hydroxy-6,7-furanocouMarin
4-hydroxy-7H-furo[3,2-g]chroMen-7-one
4-Hydroxy-7H-furo[3,2-g][1]benzopyran-7-one
Bergaptol, 98%, from Citrus medica L. var. sarcodactylis Swingle | [Molecular Formula]
C11H6O4 | [MDL Number]
MFCD00210479 | [MOL File]
486-60-2.mol | [Molecular Weight]
202.16 |
| Chemical Properties | Back Directory | [Melting point ]
287-290°C | [Boiling point ]
311.9±11.0 °C(Predicted) | [density ]
1.526±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
neat | [pka]
6.80±0.20(Predicted) | [color ]
Pale Beige to Pale Brown | [BRN ]
190104 | [LogP]
1.405 (est) |
| Hazard Information | Back Directory | [Uses]
A hydroxylated psoralen that acts as a potent inhibitors of debenzylation activity of CYP3A4 enzyme with an IC50 value of 24.92 and 42.93 μM, respectively. Recent studies suggest that it may have antiproliferative and anticancer properties. | [Definition]
ChEBI: Bergaptol is a member of psoralens and a 5-hydroxyfurocoumarin. It is a conjugate acid of a bergaptol(1-). | [Synthesis]
mL) solution in dichloromethane (CH2Cl2, 3.5 mmol) was slowly added dropwise via a glass syringe to a 1 M solution of boron tribromide (BBr3, 4 mmol) in dichloromethane. The reaction mixture was stirred at 0°C for 1-2 hours. Upon completion of the reaction, the reaction was quenched by the addition of crushed ice and the reaction mixture was extracted with dichloromethane (CH2Cl2) and washed several times with water. The organic layers were combined, dried over anhydrous sodium sulfate (Na2SO4) and concentrated to dryness under reduced pressure to give the target phenolic compound. | [in vivo]
Bergaptol (10-40 mg/kg, i.p., once a day for two weeks) improves the cognitive impairment in LPS-treated mice[4].
| Animal Model: | LPS (40 μg/kg, i.c.v.)-treated mice[4] | | Dosage: | 10-40 mg/kg | | Administration: | i.p., once a day for two week | | Result: | Reduced LPS-induced fixation and cleavage of neuronal nuclei in the CA1 region of the hippocampus (H&E staining).
Increasead the dendritic spine density of mice.
Inhibited LPS-induced neuroinflammation. |
| [target]
CYP3A4 | [IC 50]
CYP3 | [References]
[1] Bioorganic and Medicinal Chemistry Letters, 1997, vol. 7, # 20, p. 2593 - 2598
[2] Organic and Biomolecular Chemistry, 2006, vol. 4, # 8, p. 1604 - 1610
[3] European Journal of Medicinal Chemistry, 2013, vol. 60, p. 155 - 169
[4] Bioorganic and Medicinal Chemistry, 2012, vol. 20, # 2, p. 784 - 788
[5] Patent: WO2004/37827, 2004, A1. Location in patent: Page 26 |
|
|