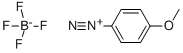| Identification | Back Directory | [Name]
4-METHOXYBENZENEDIAZONIUM TETRAFLUOROBORATE | [CAS]
459-64-3 | [Synonyms]
4-Mebenzenediazonium tetrafluoroborate
MethoxybenzenediazoniuM tetrafluoroborate
4-METHOXYBENZENEDIAZONIUM TETRAFLUOROBORATE
4-MethoxybenzenediazoniumTetrafluoroborate98% | [EINECS(EC#)]
207-296-2 | [Molecular Formula]
C7H7BF4N2O | [MDL Number]
MFCD00011897 | [MOL File]
459-64-3.mol | [Molecular Weight]
221.95 |
| Chemical Properties | Back Directory | [Appearance]
grey-brown powder | [Melting point ]
142-144 °C(lit.)
| [storage temp. ]
Refrigerator (+4°C) | [form ]
powder to crystal | [color ]
White to Orange to Green | [Water Solubility ]
Decompose in water | [λmax]
315nm(CHCl3)(lit.) | [BRN ]
3579591 | [InChI]
InChI=1S/C7H7N2O.BF4/c1-10-7-4-2-6(9-8)3-5-7;2-1(3,4)5/h2-5H,1H3;/q+1;-1 | [InChIKey]
CNKRQRKNUIYISU-UHFFFAOYSA-M | [SMILES]
[B-](F)(F)(F)F.C1(OC)=CC=C([N+]#N)C=C1 |
| Hazard Information | Back Directory | [Chemical Properties]
grey-brown powder | [Uses]
4-Methoxybenzenediazonium tetrafluoroborate has been used in the preparation of azo coupled cyclic β-enaminones. | [General Description]
Interaction of 4-methoxybenzenediazonium tetrafluoroborate with synthetic DOPA-melanin and its precursors has been studied using EPR spectroscopy and spin-trapping technique. | [Synthesis]
The general procedure for the synthesis of tetrafluoroborate-4-methoxybenzenediazonium ortho ester from hydrofluoric acid, boric acid and p-aminoanisole was as follows: first, a mixture of aqueous hydrofluoric acid (2.08 g, 104 mmol, 17.2 eq.), boric acid (2.29 g, 26.0 mmol, 4.3 eq.), and water (5 mL) was added to p-aminomethyl ether (6.0 mmol). After stirring for 5 minutes, sodium nitrite (765 mg, 11.1 mmol, 1.9 eq.) was added in portions over 3 minutes at room temperature. After 15 minutes of reaction, the precipitate formed was filtered, washed with ether (5 mL) and subsequently suspended in acetonitrile (10 mL), filtered again and dissolved in acetonitrile (4 mL). Finally, after the addition of ether, the precipitate formed was filtered and dried under reduced pressure to give pure n-phenyl tetrafluoroborate-4-methoxy diazepine. | [References]
[1] Beilstein Journal of Organic Chemistry, 2015, vol. 11, p. 1494 - 1502 |
|
|