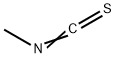| Identification | Back Directory | [Name]
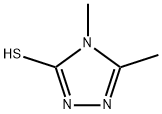
4,5-DIMETHYL-4H-(1,2,4)TRIOZOLE-3-THIOL | [CAS]
38942-50-6 | [Synonyms]
dimethyl-4H-1,2,4-triazole-3-thiol
3,4-Dimethyl-1H-1,2,4-triazole-5(4H)
3,4-Dimethyl-4H-1,2,4-triazole-5-thiol
3,4-dimethyl-1H-1,2,4-triazole-5-thione
4,5-DIMETHYL-4H-(1,2,4)TRIOZOLE-3-THIOL
4H-1,2,4-Triazole-3-thiol, 4,5-dimethyl-
4,5-Dimethyl-3-mercapto-4H-1,2,4-triazole
4,5-Dimethyl-2H-1,2,4-triazole-3(4H)-thione
3,4-Dimethyl-1H-1,2,4-triazole-5(4H)-thione
4-Methyl-5-methyl-4H-[1,2,3]triazole-3-thiol
4-triazole-3-thione,2,4-dihydro-4,5-dimethyl-3h-2
4,5-diMethyl-4,5-dihydro-1H-1,2,3-triazole-1-thiol
4,5-Dimethyl-2,5-dihydro-1H-1,2,3-triazole-1-thiol
2,4-dihydro-4,5-dimethyl-3H-1,2,4-triazole-3-thione
3H-1,2,4-Triazole-3-thione, 2,4-dihydro-4,5-dimethyl-
4,5-dimethyl-4H-1,2,4-triazole-3-thiol(SALTDATA: FREE)
3H-1,2,4-Triazole-3-thione,2,4-dihydro-4,5-dimethyl-(9CI)
4,5-Dimethyl-3-mercapto-4H-1,2,4-triazole, 4,5-Dimethyl-3-thio-4H-1,2,4-triazole, 4,5-Dimethyl-3-sulphanyl-4H-1,2,4-triazole | [EINECS(EC#)]
254-202-0 | [Molecular Formula]
C4H7N3S | [MDL Number]
MFCD01678503 | [MOL File]
38942-50-6.mol | [Molecular Weight]
129.18 |
| Chemical Properties | Back Directory | [Melting point ]
210 °C(Solv: ethanol (64-17-5)) | [Boiling point ]
150.5±23.0 °C(Predicted) | [density ]
1.35±0.1 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [pka]
9.80±0.20(Predicted) | [Appearance]
White to off-white Solid | [Sensitive ]
Stench | [InChI]
InChI=1S/C4H7N3S/c1-3-5-6-4(8)7(3)2/h1-2H3,(H,6,8) | [InChIKey]
OTVCBBXLGYYSNC-UHFFFAOYSA-N | [SMILES]
N1=C(C)N(C)C(S)=N1 |
| Hazard Information | Back Directory | [Synthesis]
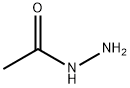
Acetylhydrazine (7.4 g, 100 mmol) was dissolved in ethanol (75 mL) in a 200 mL three-necked round-bottomed flask equipped with a magnetic stir bar and reflux condenser. To this solution was slowly added a solution of methyl isothiocyanate (7.3 g, 100 mmol) in ethanol (75 mL). The reaction mixture was stirred at reflux for 6 hours under nitrogen protection. After completion of the reaction, it was cooled to room temperature and the solvent was removed by rotary evaporator. The resulting wet solid was ground with ether and filtered to give a viscous yellow solid. This solid was further ground with hot dichloromethane/ethyl acetate (1:1, v/v) solvent mixture to give a white solid of the uncyclized intermediate. All the reaction related solids and liquids were combined and redissolved in ethanol. 1 M aqueous sodium hydroxide solution (50 mL) was added and refluxed for 1 h under nitrogen protection. The reaction solution was acidified to pH 4-5 with acetic acid and subsequently cooled in an ice bath to promote precipitation. The white solid formed was collected by filtration and washed with ether (the mother liquor was repeated the crystallization process three times). All collected solids were combined and purified by silica gel column chromatography with an eluent of 14% methanol/ethyl acetate. The collected target fractions were concentrated, washed with ether and filtered to give 5.02 g (38.9% yield) of 3,4-dimethyl-1H-1,2,4-triazole-5(4H)-thione. The product was characterized by 1H NMR (DMSO-d6): δ 2.27 (s, 3H), 3.37 (s, 3H), 13.36 (wide s, 1H). Mass spectrometry (APCI) analysis: calculated value of C4H7N3S was 129.18; measured value (M+H+) was 130.0. | [References]
[1] Patent: US2005/176701, 2005, A1. Location in patent: Page/Page column 214 |
|
|