| Identification | Back Directory | [Name]
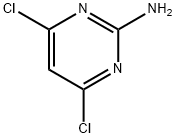
2-AMINO-4-CHLORO-6-PHENYLPYRIMIDINE | [CAS]
36314-97-3 | [Synonyms]
IFLAB-BB F2108-0197
4-Chloro-6-phenyl-2-pyrimidinamine
2-Amino-4-phenyl-6-chloropyrimidine
2-AMINO-4-CHLORO-6-PHENYLPYRIMIDINE
2-Pyrimidinamine, 4-chloro-6-phenyl-
4-Chloro-6-phenyl-pyrimidin-2-ylamine
2-AMINO-4-CHLORO-6-PHENYLPYRIMIDINE ISO 9001:2015 REACH | [Molecular Formula]
C10H8ClN3 | [MDL Number]
MFCD00030784 | [MOL File]
36314-97-3.mol | [Molecular Weight]
205.64 |
| Chemical Properties | Back Directory | [Boiling point ]
433.1±37.0 °C(Predicted) | [density ]
1.323±0.06 g/cm3(Predicted) | [storage temp. ]
under inert gas (nitrogen or Argon) at 2–8 °C | [pka]
3.02±0.10(Predicted) | [Appearance]
Off-white to light yellow Solid |
| Hazard Information | Back Directory | [Synthesis]
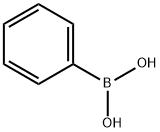
Example 2 Preparation of Compound 2 Step A - Synthesis of Compound 2b: To a reaction flask was added 2-amino-4,6-dichloropyrimidine (2.0 g, 12.2 mmol), acetonitrile (40 mL), potassium carbonate (2M aqueous solution, 6.1 mL, 12.2 mmol), tetrakis(triphenylphosphine)palladium (0.35 g, 0.31 mmol), and phenylboronic acid (0.74 g 6.1 mmol). The reaction mixture was stirred and heated to 90 °C and kept for 4 hours. After completion of the reaction, it was cooled to room temperature, transferred to a partition funnel, dichloromethane (50 mL) and water (50 mL) were added and mixed thoroughly to separate the organic layer. The aqueous layer was extracted with dichloromethane, the organic layers were combined, dried with anhydrous magnesium sulfate, filtered and concentrated. Purification by preparative thin layer chromatography (unfolding agent: 100% dichloromethane) afforded 2-amino-4-chloro-6-phenylpyrimidine (0.8 g, 64% yield). | [References]
[1] Patent: WO2009/111449, 2009, A1. Location in patent: Page/Page column 38-39
[2] Patent: WO2015/187089, 2015, A1. Location in patent: Page/Page column 65
[3] Bioorganic and Medicinal Chemistry Letters, 2004, vol. 14, # 9, p. 2303 - 2308
[4] Bioorganic and Medicinal Chemistry Letters, 2005, vol. 15, # 16, p. 3670 - 3674
[5] Bioorganic and Medicinal Chemistry Letters, 2011, vol. 21, # 8, p. 2497 - 2501 |
|
|