| Identification | Back Directory | [Name]
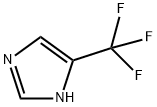
4-(Trifluoromethyl)-1H-imidazole | [CAS]
33468-69-8 | [Synonyms]
EOS-60687
4-TrifluoroMethyliMidazole
4(5)-(Trifluoromethyl)imidazole
4-(TRIFLUOROMETHYL)-1H-IMIDAZOLE
1H-Imidazole, 5-(trifluoromethyl)-
4-(TRIFLUOROMETHYL)-1H-IMIDAZOLE(I)
4-(Trifluoromethyl)-1H-imidazole 98% | [EINECS(EC#)]
206-381-1 | [Molecular Formula]
C4H3F3N2 | [MDL Number]
MFCD08458860 | [MOL File]
33468-69-8.mol | [Molecular Weight]
136.08 |
| Chemical Properties | Back Directory | [Melting point ]
149-150 | [Boiling point ]
224.7±35.0 °C(Predicted) | [density ]
1.440±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [form ]
Solid | [pka]
10.29±0.10(Predicted) | [color ]
White to Almost white | [InChI]
InChI=1S/C4H3F3N2/c5-4(6,7)3-1-8-2-9-3/h1-2H,(H,8,9) | [InChIKey]
DFLGRTIPTPCKPJ-UHFFFAOYSA-N | [SMILES]
C1NC(C(F)(F)F)=CN=1 |
| Hazard Information | Back Directory | [Chemical Properties]
Off-white crystalline powder | [Uses]
4-Trifluoromethyl-1H-imidazole acts as an inhibitor of sweet almond β-glucosidase. It is used as a reagent used in the synthesis of 3-substituted 2-aminopyridines via displacement of 3-fluoro-2-nitropyridine. | [Synthesis]
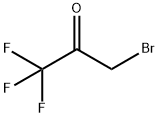
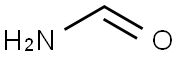
Procedure for the synthesis of 4-(trifluoromethyl)-1H-imidazole: 3-bromo-1,1,1-trifluoropropan-2-one (25.0 g, 131 mmol, Aldrich Item No. 374059) and formamide (104 mL, 2.62 mol) were sequentially added to a 500 mL round bottom flask. The reaction mixture was slowly heated to reflux temperature (about 140 °C) and kept at reflux for 2.5 hours of reaction. Upon completion of the reaction, the mixture was cooled to room temperature, diluted with 200 mL of 10% aqueous K2CO3 solution and subsequently extracted with ether (5 x 200 mL). The organic phases were combined, washed sequentially with 10% aqueous K2CO3 (2 x 100 mL) and deionized water (2 x 100 mL), dried over anhydrous MgSO4, filtered and concentrated under reduced pressure to give a brown solid. The resulting solid was washed with dichloromethane (DCM) to finalize 4-(trifluoromethyl)-1H-imidazole (4.0 g, 22% yield) as a brown solid. The structure of the product was confirmed by 1H NMR (CD3OD): δ 7.82 (s, 1H), 7.60 (s, 1H). | [References]
[1] Patent: WO2010/83246, 2010, A1. Location in patent: Page/Page column 100 |
|
|