| Identification | Back Directory | [Name]
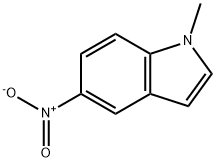
1-METHYL-5-NITRO-1H-INDOLE | [CAS]
29906-67-0 | [Synonyms]
1-Methyl-5-Nitroindole
1-METHYL-5-NITRO-1H-INDOLE
1H-Indole, 1-Methyl-5-nitro-
1-Methyl-5-nitro-1H-indole ,97% | [EINECS(EC#)]
1806241-263-5 | [Molecular Formula]
C9H8N2O2 | [MDL Number]
MFCD07772841 | [MOL File]
29906-67-0.mol | [Molecular Weight]
176.17 |
| Chemical Properties | Back Directory | [Melting point ]
167 °C | [Boiling point ]
339.2±15.0 °C(Predicted) | [density ]
1.29±0.1 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,2-8°C | [solubility ]
Chloroform (Slightly), DMSO (Slightly) | [form ]
Solid | [color ]
Dark Yellow to Very Dark Yellow | [InChI]
InChI=1S/C9H8N2O2/c1-10-5-4-7-6-8(11(12)13)2-3-9(7)10/h2-6H,1H3 | [InChIKey]
PXBQSCHRKSBGKV-UHFFFAOYSA-N | [SMILES]
N1(C)C2=C(C=C([N+]([O-])=O)C=C2)C=C1 |
| Hazard Information | Back Directory | [Chemical Properties]
Light yellow solid | [Uses]
1-Methyl-5-nitro-1H-indole is used as a reagent in the synthesis of the antiasthmatic drug Zafirlukast (Z125000). | [Synthesis]
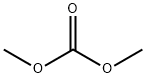
Example 14 Synthesis of 1-methyl-5-nitroindole. To a 500 mL three-necked flask equipped with a thermocouple, condenser tube, and constant pressure dropping funnel was added 5-nitroindole (20.0 g, 123 mmol), potassium carbonate (4.0 g, 29 mmol), N,N-dimethylformamide (80 mL), and dimethyl carbonate (22 mL, 261.4 mmol). The reaction mixture was heated to reflux. The reaction progress was monitored by high performance liquid chromatography (HPLC) or thin layer chromatography (TLC) (unfolding agent: heptane solution of 30% ethyl acetate). After 3 hours of refluxing, complete conversion of 5-nitroindole was confirmed by the analytical method described above. The reaction mixture was then cooled to 10±5°C and diluted with water (160 mL), at which point a yellow precipitate was generated. The mixture was continued to be stirred at room temperature for 2 h, after which the solid product was collected by filtration, washed with water (100 mL), and finally dried under high vacuum at 60-65 °C for 24 h. 1-methyl-5-nitroindole (21.1 g, 97.1% yield) was obtained as a yellow solid. | [References]
[1] Patent: US6326501, 2001, B1 |
|
|