| Hazard Information | Back Directory | [Description]
Ensetesvir fumarate (trade name Xocova) is an oral non-covalent SARS-CoV-2 main protease (Mpro) inhibitor developed by Shionogi & Co. | [Uses]
Ensitrelvir (S-217622) fumarate is the first orally active non-covalent, non-peptidic, SARS-CoV-2 3CL protease inhibitor (IC50=13 nM)[1][2]. | [Mechanism of action]
Ensetivir inhibits the replication of SARS-CoV-2 in infected patients through a once-daily dose for 5 days. Its mechanism of action is to inhibit the viral Mpro, thereby blocking a key enzyme in viral replication. | [Synthesis]
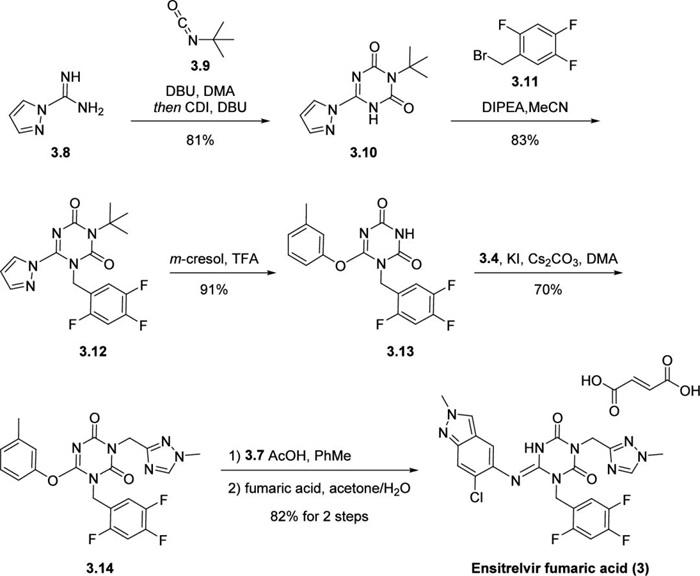
The steps to build the core structure of Ensetesvir began with the reaction of semicarbazide 3.8 with tert-butyl isocyanate, followed by the use of N,N′-carbonyldiimidazole (CDI) to ensure the formation of 1,3,5-triazinone 3.10. Next, N-alkylation with bromide 3.11 gave benzyl triazone 3.12. Under acidic conditions, pyrazole was replaced with m-cresol, which facilitated the introduction of indazole 3.7 with a minimum number of by-products in the subsequent steps of the synthesis. TFA-mediated reaction simultaneously removed the N-tert-butyl group to afford compound 3.13 in 91% yield. N-alkylation with chloride 3.4 in the presence of a base gave intermediate 3.14, which was then treated with reactive building block 3.7 in the presence of anhydrous acetic acid. Isolation of Ensetesvir fumarate was achieved by exposure to fumaric acid in water-acetone.
 | [in vivo]
Ensitrelvir fumarate dose-dependently inhibits intrapulmonary replication of SARS-CoV-2 in mice[2]. | [References]
[1] McKimm-Breschkin JL, et al. COVID-19, Influenza and RSV: Surveillance-informed prevention and treatment - Meeting report from an isirv-WHO virtual conference. Antiviral Res. 2022;197:105227. DOI:10.1016/j.antiviral.2021.105227
[2] Yuto Unoh, et al. Discovery of S-217622, a Non-Covalent Oral SARS-CoV-2 3CL Protease Inhibitor Clinical Candidate for Treating COVID-19. bioRxiv 2022.01.26.477782. |
|
|