| Identification | Back Directory | [Name]
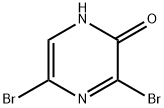
3,5-Dibromo-2-hydroxypyrazine | [CAS]
21943-15-7 | [Synonyms]
3,5-bromopyrazin-2-ol
3,5-DIBROMO-PYRAZIN-2-OL
3,5-dibromo-1H-pyrazin-2-one
3,5-dibromo-2(1H)-Pyrazinone
2-Hydroxy-3,5-dibromopyrazine
3,5-DIBROMO-2-HYDROXYPYRAZINE
2(1H)-Pyrazinone, 3,5-dibromo-
3,5-DibroMo-2-hydroxypyrazine, 95+%
3,5-Dibromo-2-hydroxypyrazine ISO 9001:2015 REACH | [Molecular Formula]
C4H2Br2N2O | [MDL Number]
MFCD07371406 | [MOL File]
21943-15-7.mol | [Molecular Weight]
253.88 |
| Chemical Properties | Back Directory | [Melting point ]
174-178°C | [density ]
2.53±0.1 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [pka]
8.35±0.60(Predicted) | [Appearance]
Light yellow to yellow Solid | [InChI]
InChI=1S/C4H2Br2N2O/c5-2-1-7-4(9)3(6)8-2/h1H,(H,7,9) | [InChIKey]
NHVGHXUOFWEOSN-UHFFFAOYSA-N | [SMILES]
C1(=O)NC=C(Br)N=C1Br |
| Hazard Information | Back Directory | [Chemical Properties]
White solid | [Uses]
3,5-Dibromo-pyrazinol ,is a pyrazine derivative that can be used as a potential building block in chemical synthesis. It can also be used in SRN1 mechanism in heteroaromatic nucleophilic substitution. | [Synthesis]
Preparation of compound 36a: 3,5-dibromopyrazin-2-ol
To a stirred solution of 3,5-dibromopyrazin-2-amine (30 g, 0.12 mol) in acetic acid (300 mL) was added sulfuric acid (50 mL) dropwise at 15-25 °C. Subsequently, a solution of sodium nitrite (16.6 g, 0.24 mol) in water (100 mL) was added dropwise to the resulting solution over a period of 1.5 hours at 10-15°C. After the dropwise addition was completed, the reaction mixture was continued to be stirred at 10-15 °C for 1 hour. The reaction mixture was poured into water (3L) and extracted with ethyl acetate (1L x 3). The organic layers were combined, washed sequentially with saturated sodium bicarbonate solution (1L×3) and brine (1L), dried with anhydrous sodium sulfate, and concentrated under reduced pressure to afford 3,5-dibromo-2-hydroxypyrazine (36a) as a yellow solid (24 g, 79.4% yield).
1H NMR (400 MHz, CDCl3): δ 7.44 (s, 1H). | [References]
[1] Patent: WO2010/16005, 2010, A1. Location in patent: Page/Page column 144 |
|
|