| Identification | Back Directory | [Name]
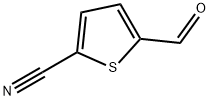
5-CYANO-2-THIOPHENE CARBALDEHYDE | [CAS]
21512-16-3 | [Synonyms]
5-CYANO-2-THIOPHENE CARBALDEHYDE
5-Cyanothiophene-2-carboxaldehyde
5-Formyl-thiophene-2-carbonitrile
5-aldehyde-2-thiophenecarbonitrile
2-Thiophenecarbonitrile, 5-formyl-
5-Cyanothiophene-2-carboxyaldehyde
5-Cyanothiophene-2-carboxaldehyde 97%
5-Cyano-2-thiophene carbaldehyde, 95+% | [Molecular Formula]
C6H3NOS | [MDL Number]
MFCD09037804 | [MOL File]
21512-16-3.mol | [Molecular Weight]
137.159 |
| Chemical Properties | Back Directory | [Melting point ]
90-95℃ | [Boiling point ]
130-135 °C(Press: 1.2 Torr) | [density ]
1.32±0.1 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
Chloroform (Slightly), Ethyl Acetate (Slightly) | [form ]
Solid | [color ]
White | [Water Solubility ]
Slightly soluble in water. |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
22-36 | [Safety Statements ]
26 | [RIDADR ]
UN 3439 6.1/PG III | [WGK Germany ]
3 | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
2934999090 |
| Hazard Information | Back Directory | [Uses]
5-Cyanothiophene-2-carboxaldehyde is found in caper and may have pest control activity against meloidogyne incognita. | [Synthesis]
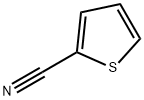
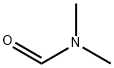
Under the protection of inert atmosphere, n-butyllithium (BuLi, 11.7 mL, 29.3 mmol, 2.5 M solution) was slowly added to a mixture containing diisopropylamine (4.1 mL, 29.3 mmol) and tetrahydrofuran (THF, 150 mL), keeping the temperature lower than -70°C. After addition, the reaction mixture was allowed to gradually warm up to room temperature. Subsequently, the mixture was cooled again to -70°C and a THF solution (15 mL) containing 2-cyanothiophene (3.0 g, 26.7 mmol) was slowly added dropwise at this temperature, and stirring was continued at -78°C for 1 hr after completion of the dropwise addition. Maintaining the reaction temperature below -70°C, N,N-dimethylformamide (DMF, 8.2 mL, 106.6 mmol) was added, followed by continued stirring at -78°C for 1.5 hours. Upon completion of the reaction, citric acid (10 g) was added to the mixture and the entire reaction mixture was poured into water. Diethyl ether extraction was carried out with the water phase and the organic phases were combined, dried and concentrated. The crude product was purified by silica gel column chromatography with an eluent gradient of 10-50% heptane/ethyl acetate (EtOAc). The final product was obtained as 5-formyl-2-thiophenecarbonitrile as a solid with a yield of 2.8 g in 76% yield. | [References]
[1] Patent: WO2011/73298, 2011, A1. Location in patent: Page/Page column 18
[2] Patent: US2008/200535, 2008, A1. Location in patent: Page/Page column 169 |
|
|