| Identification | Back Directory | [Name]
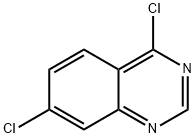
4,7-Dichloroquinazoline | [CAS]
2148-57-4 | [Synonyms]
7-dichloroquinazoline
4,7-Dichloroquizoline
4,7-DICHLOROQUINAZOLINE
Quinazoline, 4,7-dichloro-
4,7-Dichloroquinazoline
4,7-dichloro-4a,8a-dihydroquinazoline | [Molecular Formula]
C8H4Cl2N2 | [MDL Number]
MFCD00023911 | [MOL File]
2148-57-4.mol | [Molecular Weight]
199.04 |
| Chemical Properties | Back Directory | [Melting point ]
135-136 °C | [Boiling point ]
317.6±22.0 °C(Predicted) | [density ]
1.486±0.06 g/cm3(Predicted) | [storage temp. ]
under inert gas (nitrogen or Argon) at 2-8°C | [pka]
0.12±0.30(Predicted) | [Appearance]
Off-white to yellow Solid |
| Hazard Information | Back Directory | [Uses]
4,7-Dichloroquinazoline is a useful building block, used in the synthesis of aroylquinazolines via aroylation with aryl aldehydes. 4,7-Dichloroquinazoline has been identified as a dengue virus entry inhibitor. | [Synthesis]
The general procedure for the synthesis of 4,7-dichloroquinazoline from 7-chloro-4(3H)-quinazolinone was as follows: 7-chloro-3H-quinazolin-4-one (170, 0.155 g, 0.85830 mmol) was dissolved in a screw cap vial containing 1 mL of phosphorus oxychloride (POCl3). After sealing the vial, the reaction was placed in an oil bath at 100 °C for 3 hours. Upon completion of the reaction, the resulting solution was concentrated under vacuum and distilled three times by azeotropic distillation with toluene to completely remove the residual phosphorous trichloride, resulting in the target compound 4,7-dichloroquinazoline (171, 0.162 g, 0.81391 mmol, 94.828% yield). The mass spectrometry analysis showed m/z 199.0 ([M+H]+). | [References]
[1] Patent: WO2013/142613, 2013, A1. Location in patent: Page/Page column 175
[2] Synthetic Communications, 2011, vol. 41, # 24, p. 3644 - 3653
[3] Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1980, vol. 19, # 12, p. 1084 - 1087
[4] Bioorganic and Medicinal Chemistry Letters, 2001, vol. 11, # 4, p. 545 - 548
[5] Organic Letters, 2010, vol. 12, # 3, p. 552 - 555 |
|
|