| Identification | Back Directory | [Name]
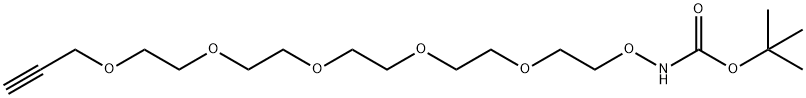
t-Boc-aminooxy-PEG5-propargyl | [CAS]
2086688-98-2 | [Synonyms]
Boc-NH-O-PEG5-Propargyl
Boc-aminooxy-PEG5-propargyl
t-Boc-aminooxy-PEG5-propargyl
3,6,9,12,15,18-Hexaoxa-2-azaheneicos-20-ynoic acid, 1,1-dimethylethyl ester | [Molecular Formula]
C18H33NO8 | [MDL Number]
MFCD30723288 | [MOL File]
2086688-98-2.mol | [Molecular Weight]
391.46 |
| Hazard Information | Back Directory | [Description]
t-Boc-aminooxy-PEG5-propargyl is a click crosslinker. Propargyl group is reactive with azide-bearing compounds or biomolecules via copper catalyzed azide-alkyne Click Chemistry to yield a stable triazole linkage. T-Boc-aminooxy can be converted to free aminooxy under mild acidic conditions and then can react with an aldehyde or ketone. The hydrophilic PEG spacer increases solubility in aqueous media. | [Uses]
Boc-aminooxy-PEG5-propargyl is a PEG-based PROTAC linker that can be used in the synthesis of PROTACs[1]. Boc-aminooxy-PEG5-propargyl is a click chemistry reagent, it contains an Alkyne group and can undergo copper-catalyzed azide-alkyne cycloaddition (CuAAc) with molecules containing Azide groups. | [IC 50]
PEGs | [References]
[1] Zhao B, et al. Protein Engineering in the Ubiquitin System: Tools for Discovery and Beyond. Pharmacol Rev. 2020 Apr;72(2):380-413. DOI:10.1124/pr.118.015651 |
|
|