| Identification | Back Directory | [Name]
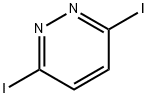
3,6-Diiodopyridazine | [CAS]
20698-04-8 | [Synonyms]
3,6-DIIDOPYRIDAZINE
3,6-Diiodopyridazine
Pyridazine, 3,6-diiodo-
3,6-Diiodopyridazine 97%
3,6-Diiodopyridazine ISO 9001:2015 REACH | [EINECS(EC#)]
623-189-7 | [Molecular Formula]
C4H2I2N2 | [MDL Number]
MFCD01004249 | [MOL File]
20698-04-8.mol | [Molecular Weight]
331.88 |
| Chemical Properties | Back Directory | [Melting point ]
155-160 °C | [Boiling point ]
382.2±22.0 °C(Predicted) | [density ]
2.765±0.06 g/cm3(Predicted) | [Fp ]
>110℃ | [storage temp. ]
2-8°C | [form ]
solid | [pka]
-0.80±0.10(Predicted) | [Appearance]
Off-white to yellow Solid | [InChI]
InChI=1S/C4H2I2N2/c5-3-1-2-4(6)8-7-3/h1-2H | [InChIKey]
GCLHXKPPHRIJOE-UHFFFAOYSA-N | [SMILES]
C1(I)=NN=C(I)C=C1 |
| Hazard Information | Back Directory | [Uses]
3,6-Diiodopyridazine is used as a reactant in the preparation of conjugated ethynyl and vinylpyridazines as rod-like conjugated molecules with light-emitting properties. | [Synthesis]
Example 12 Synthesis of compound 12: To 2 g of 3,6-dichloropyridazine was added 60 ml of acetone and 20.12 g (268.4 mmol) of sodium iodide, and the mixture was stirred under reflux conditions (bath temperature: 70 °C) for 2 hours. After completion of the reaction, water was added to the reaction mixture and extracted with ethyl acetate. The organic layer was washed sequentially with saturated aqueous sodium thiosulfate and saturated aqueous sodium chloride, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give 2.73 g (8.23 mmol) of 3,6-diiodopyridazine in 61% yield. | [References]
[1] Tetrahedron, 2015, vol. 71, # 40, p. 7670 - 7675
[2] Patent: WO2007/48802, 2007, A1. Location in patent: Page/Page column 91
[3] Journal of Heterocyclic Chemistry, 1999, vol. 36, # 5, p. 1135 - 1142
[4] Journal of Medicinal Chemistry, 1999, vol. 42, # 4, p. 669 - 676
[5] Patent: US5670492, 1997, A |
|
|