| Identification | Back Directory | [Name]
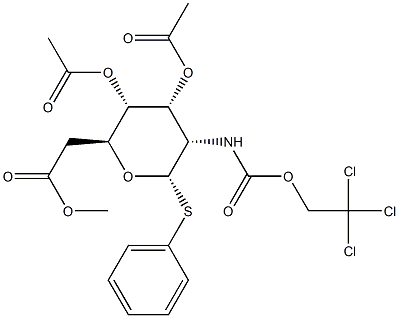
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxyformamido)-beta-D-glucopyranoside | [CAS]
187022-49-7 | [Synonyms]
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxyformamido)-&beta
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxyforMaMido)-β-D-glucopyranoside
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxyformamido)-β-D-glucopyranoside
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxyformamido)-β-D-galactopyranoside
Phenyl3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxyformamido)-β-D-glucopyranoside>
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxyformamido)-beta-D-glucopyranoside
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxycarbonylamino)-β-D-glucopyranoside
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxycarbonylamino)-β-D-glucopyranoside
Phenyl 3,4,6-Tri-O-acetyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxyformamido)-b-D-glucopyranoside, 98%
Phenyl 2-deoxy-1-thio-2-[[(2,2,2-trichloroethoxy)carbonyl]amino]-β-D-glucopyranoside 3,4,6-triacetate
Phenyl 2-deoxy-1-thio-2-[[(2,2,2-trichloroethoxy)carbonyl]amino]-β-D-glucopyranoside 3,4,6-triacetate
β-D-Glucopyranoside, phenyl 2-deoxy-1-thio-2-[[(2,2,2-trichloroethoxy)carbonyl]amino]-, 3,4,6-triacetate
(2R,3S,4R,5R,6S)-2-(Acetoxymethyl)-6-(phenylthio)-5-(((2,2,2-trichloroethoxy)carbonyl)amino)tetrahydro-2H-pyran-3,4-diyl diacetate | [Molecular Formula]
C21H24Cl3NO9S | [MDL Number]
MFCD11112181 | [MOL File]
187022-49-7.mol | [Molecular Weight]
572.84 |
| Chemical Properties | Back Directory | [Melting point ]
143°C(lit.) | [storage temp. ]
2-8°C | [form ]
powder to crystal | [color ]
White to Almost white | [InChIKey]
ODJPFIACFPEKSI-BZCSVJBRNA-N | [SMILES]
C(OC)(=O)C[C@H]1[C@H](OC(C)=O)[C@H](OC(C)=O)[C@H](NC(OCC(Cl)(Cl)Cl)=O)[C@H](SC2=CC=CC=C2)O1 |&1:5,6,11,16,26,r| |
| Hazard Information | Back Directory | [Synthesis]
GENERAL STEPS: D-Glucosamine hydrochloride (20 g, 92.8 mmol) was dissolved in water (250 mL), saturated aqueous NaHCO3 solution (250 mL) and 2,2,2-trichloroethyl chloroformate (14.05 mL, 102 mmol) were added and vigorously stirred to room temperature. The reaction mixture was filtered through a sintered funnel and the white solid was collected and dried under vacuum overnight. The resulting solid was dissolved in pyridine (100 mL), cooled to 0 °C, and acetic anhydride (100 mL) was slowly added, followed by 4-dimethylaminopyridine (DMAP, 500 mg, 4.1 mmol). The reaction solution was slowly warmed to room temperature and stirred for 12 hours. After completion of the reaction, the reaction solution was diluted with dichloromethane (200 mL), washed sequentially with 1 M HCl (3 × 100 mL), saturated aqueous NaHCO3 (2 × 100 mL) and brine (100 mL), the organic phase was dried with anhydrous Na2SO4, and the product was concentrated under reduced pressure after filtration to give the target product (2R,3S,4R,5R,6S)-2-(acetyloxymethyl )-6-(phenylthio)-5-(((2,2,2-trichloroethoxy)carbonyl)amino)tetrahydro-2H-pyran-3,4-diyl diacetate (Glucosamine 11) as a white foamy solid (44.3 g, 91% yield). The 1H NMR spectrum (400 MHz, CDCl3) of the product was in agreement with the literature report [53] with the following major signals: δ 6.23 (1H, d, J = 3.4 Hz), 5.28 (1H, dd, J = 10.7, 9.6 Hz), 5.20 (1H, t, J = 9.8 Hz), 5.14 (1H, d, J = 9.3 Hz, NHTroc ), 4.82 (1H, d, J = 12.1 Hz), 4.62 (1H, d, J = 12.1 Hz), 4.28 (1H, dd, J = 12.4, 4.0 Hz), 4.20 (1H, ddd, J = 10.7, 9.4, 3.8 Hz), 4.06 (1H, dd, J = 12.5, 2.4 Hz), 4.06- 4.01 (1H, m), 2.20 (3H, s), 2.09 (3H, s), 2.04 (6H, s). | [References]
[1] Chemical and Pharmaceutical Bulletin, 1997, vol. 45, # 2, p. 312 - 320
[2] Carbohydrate Research, 2017, vol. 452, p. 47 - 53
[3] Journal of Organic Chemistry, 2011, vol. 76, # 10, p. 3691 - 3709
[4] Angewandte Chemie - International Edition, 2014, vol. 53, # 31, p. 8190 - 8194
[5] Angew. Chem., 2014, vol. 126, # 31, p. 8329 - 8333,5 |
|
|





![α-D-Glucopyranose, 2-deoxy-2-[[(2,2,2-trichloroethoxy)carbonyl]amino]-, 1,3,4,6-tetraacetate](/CAS/20210305/GIF/122210-01-9.gif)
