[Synthesis]
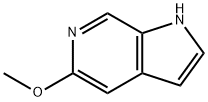
Example 12: Synthesis of 5-methoxypyrrolo[2,3-c]pyridine (Compound 9)
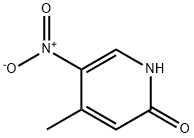
4-Methyl-5-nitro-1H-pyridin-2-one (5.00 g, 32.44 mmol) was mixed with thionyl chloride (20 ml), two drops of dimethylformamide were added under nitrogen protection and heated to reflux for 52 hours. Upon completion of the reaction, the resulting orange solution was concentrated under reduced pressure, added a small amount of anhydrous toluene and concentrated again under reduced pressure to remove residual thionyl chloride. Subsequently, the crude product was purified by column chromatography on silica gel (silica gel was pre-dried overnight at 150 °C under vacuum, about 100 g), and the eluent was dichloromethane (11). The eluate was collected and concentrated under reduced pressure to afford 2-chloro-4-methyl-5-nitropyridine (5.30 g, 30.71 mmol, 95% yield) as an orange oil, which could be crystallized by cooling to below 0 °C.
Product characterization data.
IR (CHCl3): 1605, 1550, 1520, 1450, 1360, 1345 cm-1;.
1H NMR (CDCl3): δ 9.03 (s, 1H), 7.83 (s, 1H), 2.60 (s, 3H); δ 2.60 (s, 3H).
LRMS (m/z, relative intensity): 174 (25), 173 (19), 172 (M+, 68), 157 (74), 155 (100), 128 (27), 101 (47), 100 (55), 99 (74), 90 (43), 75 (36). |