| Identification | Back Directory | [Name]
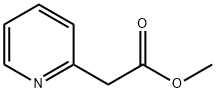
METHYL 2-PYRIDYLACETATE | [CAS]
1658-42-0 | [Synonyms]
NSC 72093
METHYLPYRIDYL-2 ACETATE
METHYL 2-PYRIDYLACETATE
METHYL 2-PYRIDINEACETATE
Methyl pyridine-2-acetate
Methyl 2-pyridinylacetate
METHYL PYRIDIN-2-YLACETATE
Methyl 2-pyridylacetate,98%
methyl 2-(pyridin-2-yl)acetate
Methyl 2-pyridylacetate, GC 98%
2-PYRIDYLACETIC ACID METHYL ESTER
2-PYRIDINEACETIC ACID METHYL ESTER
Pyridin-2-yl-aceticacidMethylester
Pyridine-2-acetic acid methyl ester
Methyl 2-Pyridineacetate
2-Pyridineacetic Acid Methyl Ester
2-Pyridylacetic Acid Methyl Ester | [EINECS(EC#)]
216-759-8 | [Molecular Formula]
C8H9NO2 | [MDL Number]
MFCD00006358 | [MOL File]
1658-42-0.mol | [Molecular Weight]
151.16 |
| Chemical Properties | Back Directory | [Appearance]
Clear yellow liquid | [Boiling point ]
103 °C0.5 mm Hg(lit.)
| [density ]
1.119 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.506(lit.)
| [Fp ]
>230 °F
| [storage temp. ]
Inert atmosphere,Room Temperature | [form ]
clear liquid | [pka]
4.35±0.12(Predicted) | [color ]
Light yellow to Amber to Dark green | [InChIKey]
ORAKNQSHWMHCEY-UHFFFAOYSA-N | [CAS DataBase Reference]
1658-42-0 |
| Hazard Information | Back Directory | [Chemical Properties]
Clear yellow liquid | [Synthesis]
The general procedure for the synthesis of methyl 2-pyridylacetate from methanol and 2-pyridylacetic acid hydrochloride was as follows: the target compound was prepared by referring to literature methods. 2.00 g of 2-pyridine acetic acid hydrochloride (11.5 mmol, 1.00 eq.) was added to a round-bottom flask with 14 mL of methanol. The reaction system was cooled to 0 °C and 2.90 mL of trimethylchlorosilane (23.0 mmol, 2.00 eq.) was slowly added dropwise. After the dropwise addition, the reaction mixture was gradually warmed to room temperature and stirred overnight. After completion of the reaction, the solvent was removed by rotary evaporator under reduced pressure. The residual solid was neutralized by slowly adding 40 mL of saturated aqueous sodium bicarbonate solution to the residual solid. Subsequently, the aqueous phase was extracted with dichloromethane (4 x 40 mL), and the organic phase was combined and dried over anhydrous sodium sulfate. After filtration, the organic phase was concentrated under reduced pressure to afford the crude product methyl 2-pyridylacetate (1.73 g, 11.47 mmol, quantitative yield) as a clear oil. The structure of the product was confirmed by comparing the spectral data of the compound with data reported in the literature. | [References]
[1] Tetrahedron, 2011, vol. 67, # 24, p. 4435 - 4441
[2] Patent: WO2017/87667, 2017, A1. Location in patent: Paragraph 0069
[3] Journal of the American Chemical Society, 2015, vol. 137, # 32, p. 10246 - 10253
[4] Journal of Organic Chemistry, 2001, vol. 66, # 20, p. 6595 - 6603 |
|
|