| Identification | Back Directory | [Name]
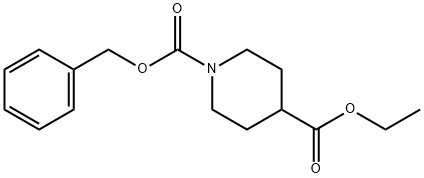
1-BENZYL 4-ETHYL PIPERIDINE-1,4-DICARBOXYLATE | [CAS]
160809-38-1 | [Synonyms]
Ethyl 1-Cbz-piperidine-4-carboxylate
1-BENZYL 4-ETHYL PIPERIDINE-1,4-DICARBOXYLATE
1-O-benzyl 4-O-ethyl piperidine-1,4-dicarboxylate
Piperidine derivative Ethyl-N-CBZ-piperidine-4-carboxylate
1,4-Piperidinedicarboxylic acid, 4-ethyl 1-(phenylMethyl) ester
piperidine-1,4-dicarboxylic acid O4-ethyl ester O1-(phenylmethyl) ester
Piperidine derivative CAS NO.160809-38-1 Ethyl-N-CBZ-piperidine-4-carboxylate | [Molecular Formula]
C16H21NO4 | [MDL Number]
MFCD06204513 | [MOL File]
160809-38-1.mol | [Molecular Weight]
291.34 |
| Chemical Properties | Back Directory | [Boiling point ]
403.7±45.0 °C(Predicted) | [density ]
1.165±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [pka]
-2.28±0.40(Predicted) | [Appearance]
Colorless to light yellow Liquid |
| Hazard Information | Back Directory | [Synthesis]
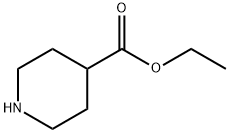
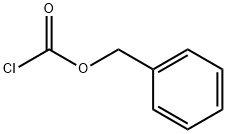
The general procedure for the synthesis of ethyl 1-Cbz-piperidine-4-carboxylate from ethyl 4-piperidinecarboxylate and benzyl chloroformate was as follows: a solution of benzyl chloroformate (95 g, 0.56 mol) in dichloromethane (200 mL) was added slowly and dropwise to a stirred ethyl 4-piperidinecarboxylate (87 g, 0.55 mol) and sodium carbonate (60 g, 0.57 mol) mixture. The dropwise addition was controlled to be completed in 70 min, during which dichloromethane (200 mL) was added simultaneously. The reaction mixture was stirred continuously at room temperature for 2.5 days, followed by filtration through a Celte pad. The filtrate was concentrated under vacuum and the residue was partitioned between 2M aqueous hydrochloric acid and ether. The organic layer was separated, dried with anhydrous magnesium sulfate, filtered and concentrated. Finally, the residue was purified by silica gel column chromatography (eluent: ethyl acetate/isohexane) to afford the target product ethyl 1-Cbz-piperidine-4-carboxylate (152 g, 94% yield). The product was characterized by 1H NMR (360 MHz, CDCl3): δ 7.40-7.27 (5H, m), 5.12 (2H, s), 4.22-3.99 (2H, m), 4.40 (2H, q, J=7.4 Hz), 2.93 (2H, br t, J=11.6 Hz), 2.45 (2H, m), 1.97-1.81 (2H, m), 1.74-1.56 (2H, m), 1.25 (3H, t, J=7.4 Hz). | [References]
[1] Patent: WO2004/78750, 2004, A1. Location in patent: Page 64
[2] Patent: US2006/229289, 2006, A1. Location in patent: Page/Page column 21
[3] Patent: WO2007/93603, 2007, A1. Location in patent: Page/Page column 15
[4] Patent: EP1522540, 2005, A1. Location in patent: Page/Page column 63
[5] Patent: WO2015/118342, 2015, A1. Location in patent: Page/Page column 67; 68 |
|
| Company Name: |
JSK Chemicals
|
| Tel: |
+919879767970 |
| Website: |
www.jskchemicals.com |
|