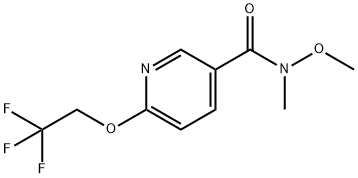
| Identification | Back Directory | [Name]
6-(2,2,2-TRIFLUORO-ETHOXY)-PYRIDINE-3-CARBALDEHYDE | [CAS]
159981-19-8 | [Synonyms]
6-(2,2,2-Trifluoroethoxy)nicotinaldehyde
6-(2,2,2-trifluoroethoxy)-3-pyridinecarboxaldehyde
6-(2,2,2-TRIFLUORO-ETHOXY)-PYRIDINE-3-CARBALDEHYDE
3-Pyridinecarboxaldehyde, 6-(2,2,2-trifluoroethoxy)- | [Molecular Formula]
C8H6F3NO2 | [MDL Number]
MFCD06738679 | [MOL File]
159981-19-8.mol | [Molecular Weight]
205.13 |
| Chemical Properties | Back Directory | [Boiling point ]
239.8±40.0 °C(Predicted) | [density ]
1.356±0.06 g/cm3(Predicted) | [storage temp. ]
under inert gas (nitrogen or Argon) at 2-8°C | [pka]
0.97±0.22(Predicted) | [Appearance]
White to off-white Solid |
| Hazard Information | Back Directory | [Synthesis]
General method: (2) Diisobutylaluminum hydride (DIBAL-H, 1.01 mol/L solution in toluene, 1.95 mL, 1.97 mmol) was added slowly and dropwise to a solution in toluene (11 mL) of N-methoxy-N-methyl-6-(2,2,2-trifluoroethoxy)pyridine-3-carboxamide (432 mg, 1.64 mmol) under nitrogen protection , the reaction temperature was -78 °C. After dropwise addition, the reaction was kept at -78 °C with continued stirring for 1 hour. Subsequently, the reaction was quenched by slow dropwise addition of methanol (5 mL) to the reaction mixture and gradually warmed up to room temperature. A 50% aqueous solution of Rochelle salt (potassium sodium tartrate) (10 mL) was added and stirred for 1.5 hours at room temperature. After completion of the reaction, the mixture was extracted with chloroform, the organic layers were combined and concentrated under reduced pressure. The crude product obtained was purified by silica gel column chromatography with the eluent ratio of (hexane:ethyl acetate= 9:1→7:3) to afford the target product 6-(2,2,2-trifluoroethoxy)pyridine-3-carbaldehyde as a colorless solid (287 mg). The structure of the product was confirmed by 1H NMR (300 MHz, CDCl3): δ 4.86 (q, J = 8.4 Hz, 2H), 6.97-7.02 (m, 1H), 8.16 (dd, J = 8.6, 2.3 Hz, 1H), 8.62-8.66 (m, 1H), 10.00 (d, J = 0.6 Hz, 1H).LCMS analysis: Retention time 1.86 min (condition 3), MS ESI/APCI Dual posi: 206 [M + H]+. | [References]
[1] Patent: EP2687507, 2014, A1. Location in patent: Paragraph 0379 |
|
| Company Name: |
Tetranov Biopharm
|
| Tel: |
13526569071 |
| Website: |
http://www.leadmedpharm.com/index.html |
| Company Name: |
SynAsst Chemical.
|
| Tel: |
021-60343070 |
| Website: |
www.yuhua99.com/ShowSupplierProductsList15848/0_EN.htm |
|