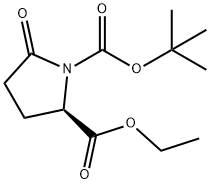
| Identification | Back Directory | [Name]
1-BOC-D-PYROGLUTAMIC ACID ETHYL ESTER
| [CAS]
144978-35-8 | [Synonyms]
Boc-D-Pyrog
144978-35-8
Boc-D-PyrogL
BOC-D-Pyr.Oet
Ethyl Boc-D-Pyroglutamate
Ethyl N-Boc-D-pyroglutamate
N-Boc-D-pyroglutamic Acid Ethyl
BOC-D-PYROGLUTAMIC ACID ETHYL ESTER
N-Boc-D-pyroglutamic acid ethyl ester
1-BOC-D-PYROGLUTAMIC ACID ETHYL ESTER
Ethyl N-(tert-Butoxycarbonyl)-D-pyroglutaMate
Ethyl (R)-N-(tert-butoxycarbonyl)pyroglutaMate
EthylN-(tert-Butoxycarbonyl)-D-pyroglutamate>
1-BOC-D-PYROGLUTAMIC ACID ETHYL ESTER USP/EP/BP
1-BOC-D-PYROGLUTAMIC ACID ETHYL ESTER 144978-35-8
N-(tert-Butoxycarbonyl)-D-pyroglutamic Acid Ethyl Ester
(R)-1-Tert-butyl 2-ethyl 5-oxopyrrolidine-1,2-dicarboxylate
1-tert-butyl 2-ethyl (2R)-5-oxopyrrolidine-1,2-dicarboxylate
1-(tert-butyl) 2-ethyl (R)-5-oxopyrrolidine-1,2-dicarboxylate
Ethyl (R)-1-(tert-Butoxycarbonyl)-5-oxopyrrolidine-2-carboxylate
1-O-tert-butyl 2-O-ethyl (2R)-5-oxopyrrolidine-1,2-dicarboxylate
Ethyl N-(tert-Butoxycarbonyl)-D-pyroglutamate
(R)-1-(tert-Butoxycarbonyl)-5-oxopyrrolidine-2-carboxylic Acid Ethyl Ester
(R)-5-Oxo-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester 2-ethyl ester
1,2-Pyrrolidinedicarboxylic acid, 5-oxo-, 1-(1,1-dimethylethyl) 2-ethyl ester, (2R)- | [Molecular Formula]
C12H19NO5 | [MDL Number]
MFCD09261329 | [MOL File]
144978-35-8.mol | [Molecular Weight]
257.283 |
| Chemical Properties | Back Directory | [Melting point ]
54.0 to 58.0 °C | [Boiling point ]
375.0±35.0 °C(Predicted) | [density ]
1.182±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [form ]
powder to crystal | [pka]
-4.15±0.40(Predicted) | [color ]
White to Almost white | [Optical Rotation]
Consistent with structure | [InChI]
InChI=1S/C12H19NO5/c1-5-17-10(15)8-6-7-9(14)13(8)11(16)18-12(2,3)4/h8H,5-7H2,1-4H3/t8-/m1/s1 | [InChIKey]
YWWWGFSJHCFVOW-MRVPVSSYSA-N | [SMILES]
N1(C(OC(C)(C)C)=O)C(=O)CC[C@@H]1C(OCC)=O |
| Hazard Information | Back Directory | [Uses]
1-Boc-D-Pyroglutamic acid ethyl ester is a crucial compound that could be used as a pharmaceutical intermediate in pharmaceutical synthesis and scientific research. It could synthesise (2S,5S)-N-Boc-5-methylpyrrolidine-2-carboxylic acid.
| [Synthesis]
An acetonitrile (150 mL) solution of di-tert-butyl dicarbonate (30.5 g, 0.14 mol) was slowly added to a reaction vial containing ethyl (S)-5-oxopyrrolidine-2-carboxylate (20 g, 0.127 mol) and di-tert-butyl dicarbonate (30.5 g, 0.14 mol) at 0 °C. 0.013 mol) in a reaction vial. The reaction mixture was stirred overnight at room temperature and then concentrated under vacuum. The residue was purified by fast chromatography (eluent: 30% ethyl acetate/hexane) to afford ethyl Boc-D-pyroglutamate (163A, 32.5 g, 100%) as an oil.1H NMR (CDCl3, 400 MHz) δ 4.58 (1H, dd, J = 3.0,9.5Hz), 4.22 (2H, q, J = 7.3Hz), 4.22 (2H, q, J = 7.3Hz), 4.22 (2H, q, J = 7.3Hz), 2.56-2.66 (2H, q, J = 7.3Hz). 2.56-2.66 (1H, m), 2.48 (1H, ddd, J = 3.8,9.6,13.1Hz), 2.25-2.35 (1H, m), 1.97-2.04 (1H, m), 1.48 (9H, s) and 1.28 (3H, t, J = 7.3Hz). | [References]
[1] Patent: US2008/9497, 2008, A1. Location in patent: Page/Page column 70-71
[2] Patent: EP2272825, 2015, B1. Location in patent: Paragraph 0138; 0139
[3] Organic Preparations and Procedures International, 2001, vol. 33, # 4, p. 405 - 409
[4] Organic and Biomolecular Chemistry, 2006, vol. 4, # 21, p. 3894 - 3897
[5] Patent: US2011/34438, 2011, A1. Location in patent: Page/Page column 25-26 |
|
|