| Identification | Back Directory | [Name]
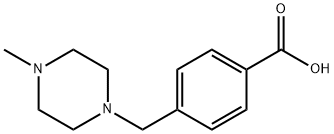
4-(4-Methylpiperazin-1-ylmethyl)benzoic acid | [CAS]
106261-48-7 | [Synonyms]
106261-48-7
Imatinib Imp.S
Imatinib-int G
Imatinib Impurity 36
Imatinib Impurity 42
Imatinib impurities5
4-(4-Methyl-1-piperazinylmethyl)benzoicAci
4-(4-Methyl-1-piperazinylMethyl)benzoic Acid
4-(4-METHYLPIPERAZIN-1-YLMETHYL)BENZOIC ACID
4-[(1-methyl-2-piperazinyl)methyl]benzoic acid
4-(4-Methyl-1-piperazinylmethyl)benzoicacid,97%
4-[(4-methylpiperazin-4-ium-1-yl)methyl]benzoate
Benzoic acid,4-[(4-methyl-1-piperazinyl)methyl]-
4-[(4-Methyl-1-piperaziny)Methyl]benzoic acid dihy
4-[(4-Methyl-1-piperaziny)methyl]benzoic acid dihydrochloride
Imatinib Related Compound (4-(4-Methylpiperazin-1-yl)methyl)benzoic Acid) | [EINECS(EC#)]
1312995-182-4 | [Molecular Formula]
C13H18N2O2 | [MDL Number]
MFCD01632530 | [MOL File]
106261-48-7.mol | [Molecular Weight]
234.29 |
| Chemical Properties | Back Directory | [Melting point ]
310-312 °C | [Boiling point ]
377.2±32.0 °C(Predicted) | [density ]
1.174±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [pka]
4.31±0.10(Predicted) | [Appearance]
White to off-white Solid | [Water Solubility ]
Slightly soluble in water. |
| Hazard Information | Back Directory | [Uses]
4-(4-Methyl-1-piperazinylmethyl)benzoic acid is used as Imatinib intermediate, as pharmaceutical intermediate. | [Synthesis]
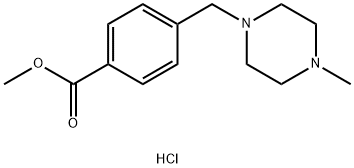
GENERAL STEPS: Methyl 4-(4-methylpiperazin-1-ylmethyl)benzoate hydrochloride (1.25 g) was dissolved in potassium hydroxide-methanol solution (0.93 g KOH dissolved in 15 ml methanol). Water (0.75 ml) was added and the mixture was heated to reflux for 1 hour. After completion of the reaction, the mixture was cooled to room temperature and the pH was adjusted to 6 with 2 M hydrochloric acid solution. the solvent was removed by evaporation and the residue was dried under vacuum. The resulting residue contained 4-(4-methylpiperazin-1-ylmethyl)benzoic acid and the inorganic salt, which could be used in the subsequent reaction without further purification, assuming 100% yield of the title compound.1H NMR (400 MHz, d6-DMSO) δ: 2.27 (s, 3H), 2.44 (bs, 2H), 3.18-3.95 (m, 8H), 7.41 ( d, 2H, J=8Hz), 7.89 (d, 2H, J=8.1Hz). | [References]
[1] Patent: WO2004/63191, 2004, A1. Location in patent: Page 74 |
|
|